Chemistry Homework Assignment #2
Kotz & Treichel: Chapter 15
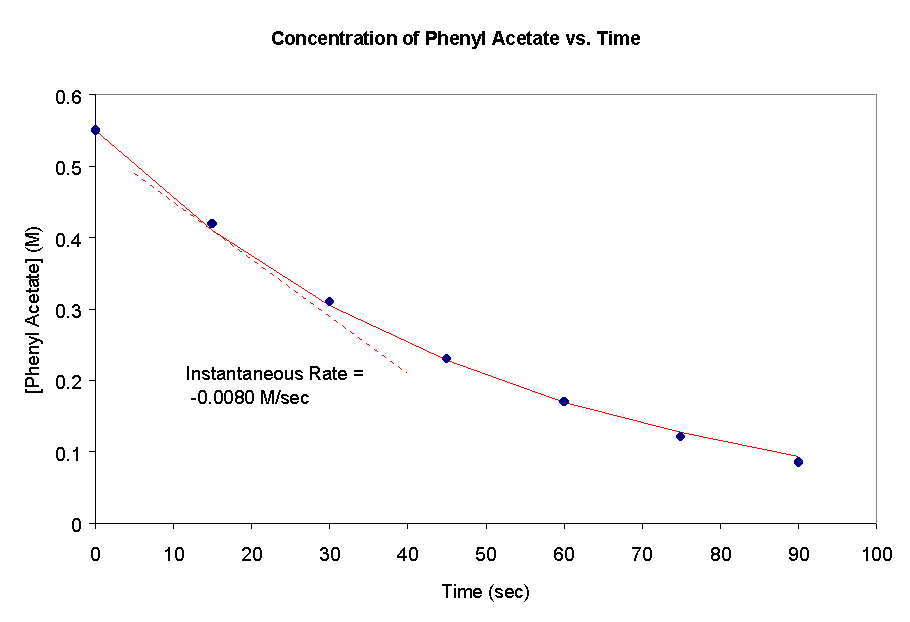
21a) The data provided is plotted below. This is an example of an exponential
decay.

b) The average rate of change of the phenyl acetate concentration between 15.0 and 30.0 seconds is -0.0073 M/sec. Between 75.0 and 90.0 seconds the rate of change is -0.0023 M/sec. This indicates that reaction is slowing down as the reactant is consumed.c) The rate of change of the phenol concentration would be equal in magnitude but opposite in sign as that for the phenyl acetate (because there is a 1:1 stoichiometric relationship). Thus it is 0.033 M/sec.
d) The instantaneous rate of the reaction is equal to the slope of the tangent line. At 15 seconds, this is -0.0080 M/sec.
25a) For the reaction: 2 NO + 2 H2 ®
N2 + 2 H2O, the rate data shows that the reaction
is second order in NO and first order in H2.
b) The rate law is, reaction rate = k[NO]2[H2].c) k = 6.32 M-2sec-1.
d) When [NO] = 0.350 M and [H2] = 0.205 M, the reaction rate (defined as the rate of appearance of N2) is 0.159 M/sec.
29) Using the integrated rate equation for a first order reaction,
the time required can be found by:
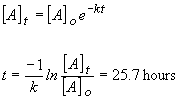
35) For a half-life of 30 minutes, the corresponding rate constant is
0.023 min-1. Using the same approach as in problem 29, the time
required to decrease the Xe(CF3)2 mass from 7.50
mg to 0.25 mg is 150 min (five half-lives).
39) The first and second order plots for the data in problem 21 are
shown below.
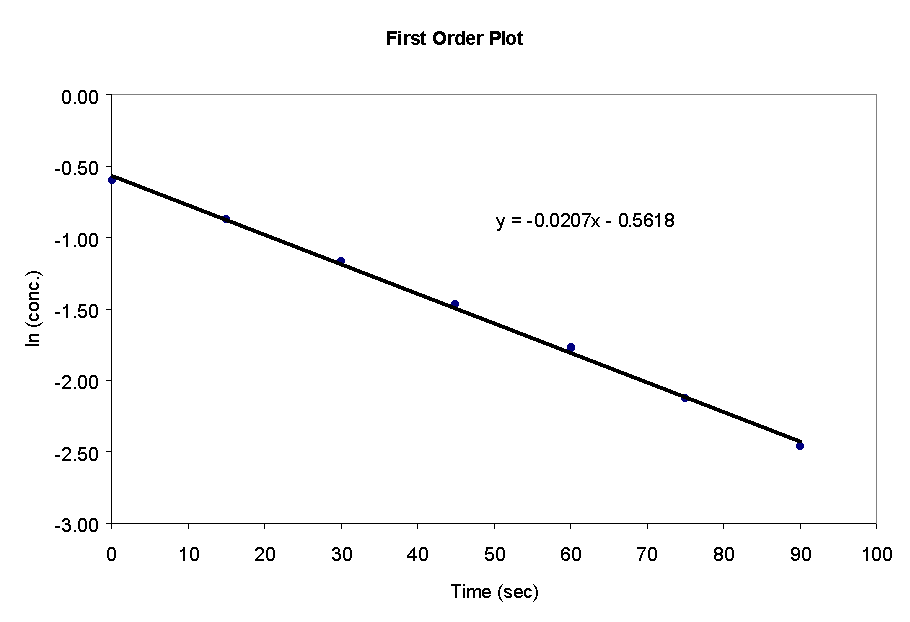
The first order plot is linear, thus the reaction is first order in phenyl acetate. The rate constant, k, is equal to the slope multiplied by -1. Thus k = 0.021 sec-1.
43) The activation energy of a reaction can be determined by measuring
the rate constant at two or more temperatures. Using two data points, the
following relationship, which is derived from the Arrhenius Equation (p.
715), is used:
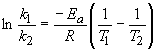
Since we know that k2 = 3k1, the
left-hand side is simply ln (3). R is the gas constant, 8.314
J/(K mol). Solving for Ea, we get 85 kJ/mol.
47a) The reaction is endothermic.
b) The reaction coordinate diagram indicates that the reaction occurs in two steps. The valley in the middle of the plot is indicative of the formation of an intermediate species.
49) The rate laws for the elementary reaction steps listed would
be:
a) rate = k [Cl] [ICl]
c) rate = k [NO2]2
b) The rate law for the rate determining step would be: rate = k2 [O3] [O]53b) step 1: bimolecular
c) To be consistent with this mechanism, the observed rate law must be,
55) Yes, the observed rate law is consistent with the mechanism proposed.
This is because the rate law for the rate determining step is: Rate = k3[IH2][I].
By recognizing that [IH2] = K2[I][H2]
and [I]2 = K1[I2], and making the
appropriate substitutions, the following rate law is obtained: Rate = K1K2k3[I2][H2],
or kobs [I2][H2]. Here, kobs
is the observed rate constant for the reaction and is equal to K1K2k3.
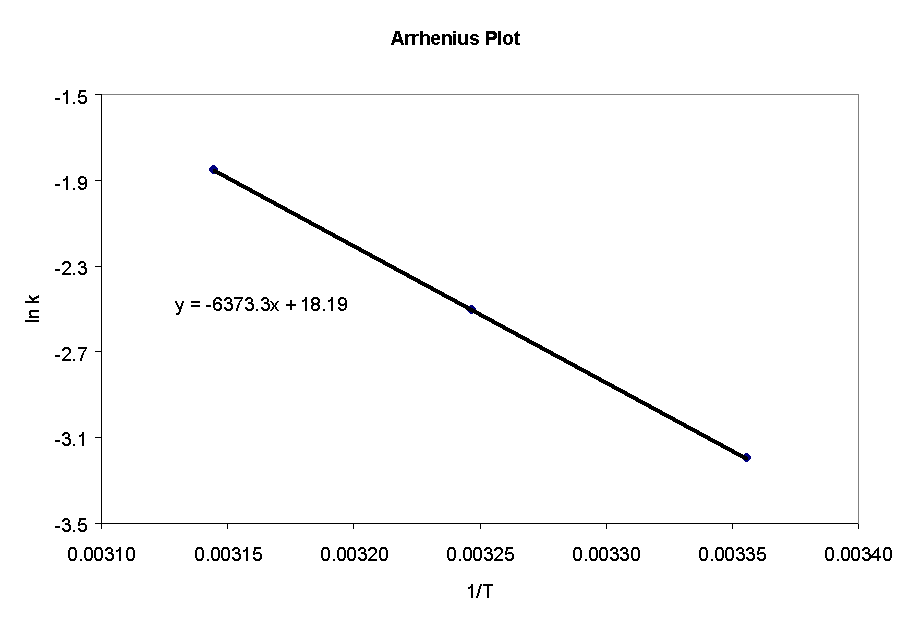
81) The Arrhenius Plot of the data is shown below. From the slope of
the line (slope = Ea/R), the activation energy is found
to be: Ea = 53.8 kJ/mol.
85) The rate law predicted by this mechanism is: ![]() ,
or simply,
,
or simply,
![]() .
.
The reaction is first order in HA, thus if the HA concentration is doubled, the reaction rate will also double.