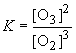
Chemistry Homework Assignment #3
Kotz & Treichel: Chapter 16
2) a) false b) true
c) false d) true e) false
5) If Q < K, the reaction will shift to the right (toward the products)
and reactant will be consumed until equilibrium is established.
7 a) The equilibrium will shift to the right if temperature is reduced.
b) The equilibrium will shift to the left (toward
reactants) if volume is increased.
9a)
b)
c) K = [H2O] [CO2] [NH3]2
d) K = [Ba2+] [SO42-]
11)
13) For this reaction,
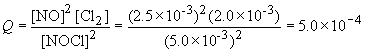
Since Q < K, the reaction will proceed to the right until equilibrium is established.
27) For the reaction provided, the equilibrium expression is:
![]()
Making the table of changes, we have:
| CO | Cl2 | COCl2 | |
| Initial | 0.0102 | 0.00609 | 0 |
| Change | -x | -x | x |
| Final | 0.0102 - x | 0.00609 - x | x |
Since the final concentration of chlorine is given, we can write 0.00301 = 0.00609 - x. So, x = 0.00308.
Substituting into the equilibrium concentration values in the above table gives the following results:
[CO]eq = 0.00712 M
[COCl2]eq = 0.00308 M
Using the equilibrium expression, K can be calculated as:
![]()
37) For the reaction provided, the equilibrium expression is:
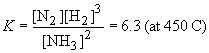
The table of equilibrium changes is:
| NH3 | N2 | H2 | |
| Initial | 1.80 | 0 | 0 |
| Change | -2x | x | 3x |
| Final | 1.8-2 x | x | 3x |
This results in the polynomial 0 = 20.41 - 45.36x + 25.2 x2 -27x4, which is pretty damn ugly but can be solved graphically. The only root that makes physical sense is x = 0.567. Thus, the equilibrium concentrations are:
[NH3] = 0.666 M
[N2] = 0.567 M
[H2] = 1.701 M
The total number of gas moles per liter is then 2.934.
Using the ideal gas law, PV = nRT, the total pressure is found
to be 174 atm.
39. The shifts in the equilibrium position will be as follows:
a) to the left (you are adding more product)50. In the reaction, 2 HA = (HA)2, where HA is the acid and (HA)2 is the dimer, the equilibrium expression is:b) to the left (you are removing reactant)
c) to the left (you are decreasing the temperature of an endothermic process)
d) to the right (the right side has more gaseous species and will compensate for the lowered pressure)
![]()
To determine the amount of acid converted to dimer, we need to determine
the equilibrium concentration of the acid. This is done in the usual manner
as shown below.
| HA | (HA)2 | |
| Initial | 5.4 x 10-4 | 0 |
| Change | -2x | x |
| Final | 5.4 x 10-4 - x | x |
Substituting into the equilibrium expression, we can determine that x = 2.28 x 10-4. Thus the equilibrium concentration of the acid is 8.4 x 10-5, which represents an 84.4% conversion rate of the acid monomer.
Since the process is exothermic, an increase in temperature will decrease
the value of the equilibrium constant.
53. To convert between Kc and Kp you can convert all concentrations (mol/L) into pressure (atm) using the ideal gas law, PV = nRT. Alternatively, you can use the expression Kp = Kc(RT)Dn, where Dn is the change in the number of gaseous molecules in the balanced reaction (this relationship is derived on page 753). Since the balanced reaction has 2 gas molecules on the left and 3 on the right, Dn is equal to one. Using the other information in the question we have the following:
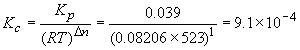
61. The equilibrium expression for the reaction is:
![]()
To find the equilibrium concentrations, we set up the following table:
| SO2Cl2 | SO2 | Cl2 | |
| Initial | 0.0497 | 0 | 0 |
| Change | -x | x | x |
| Final | 0.0497 - x | x | x |
Solving for x (x = 0.0298) and substituting into the final concentrations above yields,
[SO2Cl2] = 0.0199 M (60% dissociated)[SO2] = [Cl2] = 0.0298 M
b) To do this problem, you need to convert the pressure of chlorine
to concentration if you want to use the Kc provided.
Using the ideal gas law, 1.00 atm at 375°
C is equivalent to 0.0188 mol/L (because n/V = P/RT; R = 0.08206
(L-atm)/(K-mol) and T = 648 K). Making a new table and solving for
x, you get:
| SO2Cl2 | SO2 | Cl2 | |
| Initial | 0.0497 | 0 | 0.0188 |
| Change | -x | x | x |
| Final | 0.0497 - x | x | 0.0188 + x |
Here, x = 0.0251, therefore:
[SO2Cl2] = 0.0246 M (49% dissociated)c) This is expected from LeChâtelier's Principle. Since you are adding product, the equilibrium will shift to the reactant side, resulting in less dissociation of SO2Cl2.[SO2] = 0.0251 M
[Cl2] = 0.0439 M