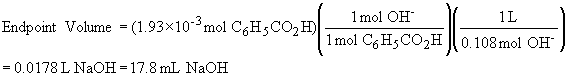
Chemistry Homework Assignment #5
Kotz & Treichel: Chapter 18
17) In this problem, benzoic acid (0.235 g, 1.93 ´ 10-3 mol) is dissolved in 100 mL, and titrated with 0.108 M NaOH. To find the concentrations at the equivalence point, we must first calculate what the equivalence point is…..
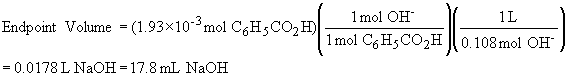
Thus, the total solution volume at the endpoint of the titration is 117.8 mL.
The concentration of Na+ is simply the number of moles of Na+ delivered to the solution divided by the total volume:
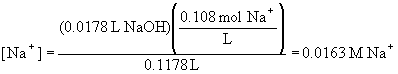
The benzoate ion concentration is:
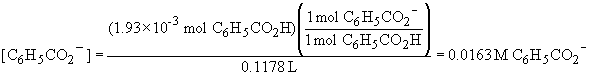
To find the hydroxide and hydronium ion concentrations, we need to consider the following equilibrium,
C6H5CO2- + H2O « C6H5CO2H + OH-
| C6H5CO2- (M) | C6H5CO2H (M) | OH- (M) | |
| Initial | 0.0163 | 0 | 0 |
| D | -x | x | x |
| Final | 0.0163-x | x | x |
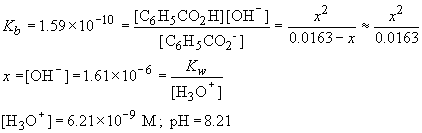
25. This problem involves the preparation of a buffer consisting of
lactic acid (0.0500 mol) and its conjugate base, sodium lactate (2.75 g,
0.02455 mol). The Ka of lactic acid is 1.4 ´
10-4. The pH can be obtained using the Henderson-Hasselbach
equation as follows:
![]()
Note that we do not need to calculate the concentration of the base;
using the number of moles directly yields the same result.
27) To answer this question, we need to satisfy the following equation, where x is the number of moles of acid (ammonium chloride) that needs to be added to the base solution:
![]()
![]()
0.0893 moles of NH4Cl has a mass of 4.77 g.
39a) pKa = 7.21 (this is obtained from Ka2 of H3PO4)
b) Using the Henderson-Hasselbach equation, we have
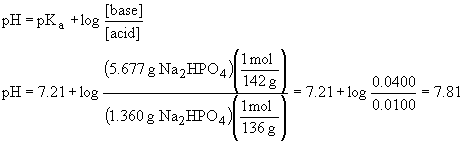
43) This problem did not include a volume of acid, which would be necessary
to calculate the pH at various points during the titration. Let's assume
the volume of acid is 25.00 mL.
a) Before any NaOH is added, what you have is a simple solution of HCN. This is solved in the usual way for a weak acid problem. The reaction is:
| HCN (M) | CN- (M) | H3O+ (M) | |
| Initial | 0.050 | 0 | 0 |
| D | -x | x | x |
| Final | 0.050-x | x | x |
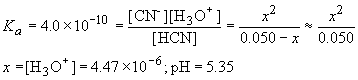
b) For the titration of any weak acid (or weak base) the pH at the mid-point
(50% of the equivalent volume) is equal to the pKa of the acid (or conjugate
acid). You should be able to show why this is true by using the Henderson-Hasselbach
equation. Therefore, the pH at the mid-point will be 9.40.
c) Since this problem involves calculating the pH of a solution that contains both the acid and its conjugate base, the Henderson-Hasselbach equation can be used. To do so, the number of moles of acid and conjugate base need to be determined. The neutralization reaction is:
HCN + OH- ® CN- + H2O
The number of moles acid present initially is 1.25´
10-3. At 95% of the required NaOH, 95% of the acid has been
converted to conjugate base, cyanide (CN-). Thus the number
moles of acid is (0.05)( 1.25´ 10-3)
and the number of moles of cyanide is
(0.95)( 1.25´ 10-3). Therefore,
![]()
d) The volume of base required to reach the equivalence point is:
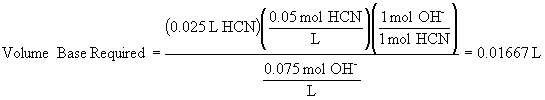
e) At the equivalence point, all of the acid has been converted to its
conjugate base. So, to find the pH at the equivalence point, you need to
find the concentration of the conjugate base (CN-) and approach
the problem as a typical weak base problem. The concentration of CN-
is given by the moles of CN- (1.25´
10-3, the same number of moles of HCN that you started with)
divided by the total volume (41.67 mL, initial volume of acid plus the
volume of base you added). The concentration of CN- is then
0.0300 M. The equilibrium reaction between CN- and water is:
CN- + H2O ® HCN + OH-
| CN- (M) | HCN (M) | OH- (M) | |
| Initial | 0.0300 | 0 | 0 |
| D | -x | x | x |
| Final | 0.0300-x | x | x |
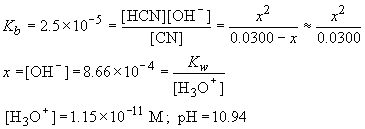
f) Alizarin Yellow would be a suitable indicator.
g) At 105% of the required base (17.50 mL), 1.31 ´
10-3 mol of OH- have been added
(1.05)(1.25´ 10-3). The
excess hydroxide is found by:
| HCN (moles) | OH- (moles) | CN- (moles) | |
| initial | 1.25 x 10-3 | 1.31 x 10-3 | 0 |
| D | -1.25 x 10-3 | -1.25 x 10-3 | 1.25 x 10-3 |
| final | 0 | 0.06 x 10-3 | 1.25 x 10-3 |
The concentration of hydroxide is then, 1.47 ´
10-3, which has a pH of 11.17.
| B (M) | BH+ (M) | OH- (M) | |
| Initial | 0.0256 | 0 | 0 |
| D | -x | x | x |
| Final | 0.0256-x | x | x |
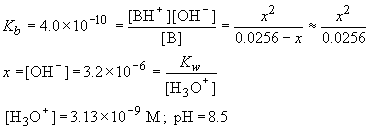
b) The equivalence point is:
Therefore the concentration of the conjugate acid at the equivalence point is 1.02´ 10-2 M. So,
| BH+ (M) | B (M) | H3O+ (M) | |
| Initial | 0.00102 | 0 | 0 |
| D | -x | x | x |
| Final | 0.00102-x | x | x |
c) At the midpoint, pH = pKa = 4.60
d) 2,4-dinitrophenol would be a good indicator.
e) Use the Henderson-Hasselbach for all of these volumes of acid added. You need to first find the number of moles of base and conjugate acid present....
i) 5.00 mL acid added
| B (moles) | H3O+ (moles) | BH+ (moles) | |
| initial | 6.4 x 10-4 | 9.75 x 10-5 | 0 |
| D | -9.75 x 10-5 | -9.75 x 10-5 | 9.75 x 10-5 |
| final | 5.43 x 10-4 | 0 | 1.25 x 10-3 |
For the remaining volumes of acid added the same approach is used.
ii) 10.0 mL acid added
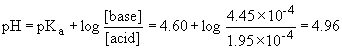
iii) 15.0 mL acid added
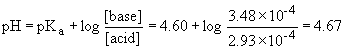
iv) 20.0 mL acid added
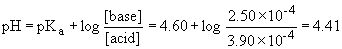
v) 24.0 mL acid added
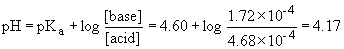
vi) 30.0 mL acid added
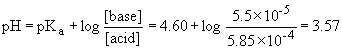
The resulting curve is:
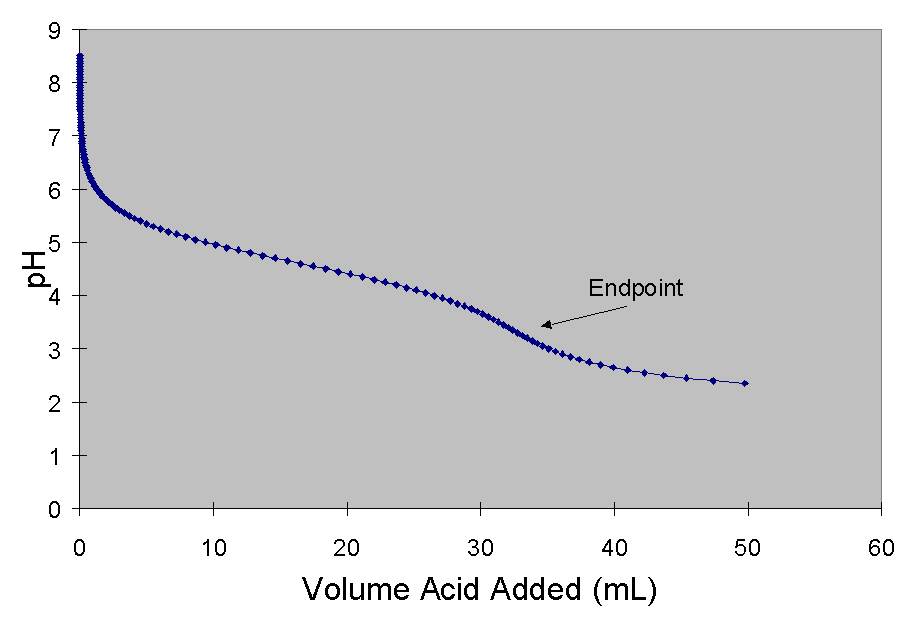
63a) Figger 1 depicts the pH changes that may be observed when a weak acid is titrated with a strong base. How do I know this? Aaaaah, velly simple. The pH before any titrant is added is less than 7 (bing! An acid.). The pH then rises briefly, then reaches a plateau (the buffered region). Since only strong acids do not form buffered solutions, the acid must have been a weak acid. In fact, from the figure I figger that the Ka of the acid is approximately 8 ´ 10-5.
b) This figger depicts the titration of a strong base with a strong
acid. Note that there is no buffered region in this titration curve, the
presence of which would indicate that the base was weak. Wait… there's
more. I'm sensing that the initial concentration of the base was 0.003
M. Yes, that's it.
c) This figger corresponds to the titration of a strong acid (~0.06
M) with a strong base.
67) As you breath in more CO2 (the concentration of which will increase in the bag), more CO2 is dissolved in the blood. This reacts to form carbonic acid, which will decrease the pH of the blood.
By taking deep breaths and exhaling deeply, you remove CO2
from your lungs more effectively. Because the reactions are all reversible,
by LeChâtelier's Principle, a decrease in the partial pressure of
CO2 in your lungs will decrease the amount of carbonic acid
in your blood. This will, in turn, reduce the hydronium ion concentration
and increase the pH.
Additional Problems:
1) This problem requires the use of charge balance to accurately determine pH. This problem is unlike other strong acid or strong base problems in which the concentration of the acid or base is relatively high. In those cases, you can neglect the autoionization of water when calculating the pH. In this problem, the concentration of base is so low that the hydroxide ion concentration that exists due to the dissolved base in comparable to that in pure water. To determine the equilibrium concentration of hydroxide we need to use the charge balance:
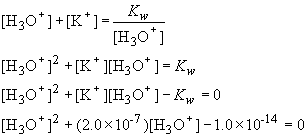
Solving the quadratic, we obtain [H3O+] = 1.2
´
10-8 M. Thus pH = 7.92.
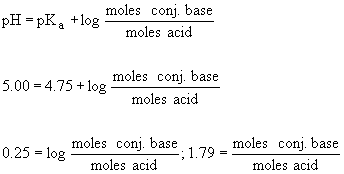
Thus the ratio of acetate to acetic acid is 1.79:1. Note that we have
no way of calculating the absolute concentration of either species. We
can only determine the ratio using the Henderson-Hasselbach equation.
3) This is a simple "plug-in" problem that illustrates why the assumption that the sum of the charges (i.e., the net charge) in a solution is always exceedingly small (assumed to be zero).
First, to convert charge in molarity to coulombs, we have:
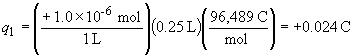
By the same relationship, q2 = -0.024 C. The force between the beakers is then:
![]()