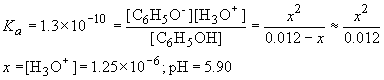= + 21.36 J K-1mol-1
![]()
DSuniverse = DSsystem
+ DSsurroundings = 1205 J
K-1mol-1
Chemistry Homework Assignment #7
Kotz & Treichel: Chapter 20
4) a) True
b) False. "Exothermic reactions are always spontaneous if DS° sys is positive."c) False. "Reactions with positive DH° rxn and DS° rxn are spontaneous at high temperature".
d) True
e) False. "When the equilibrium constant of a reaction is less than 1, the DG° rxn is greater than zero."
9) a) MgCl2 has the
higher entropy. (Both are crystalline solids, but more complex materials
tend to have greater
entropy than simpler materials.)
b) (CH3)2NH2 (g) has the greater entropy since it is a gas.c) Hg (l) has the greater entropy since it is a liquid.
11) a) NH4Cl(s) ®
NH4Cl (aq)
DS° = 169.9 J K-1mol-1 - 94.6 J K-1mol-1 = 75.3 J K-1mol-1
b) C2H5OH (l) ® C2H5OH (g)DS° = 282.7 J K-1mol-1 - 160.7 J K-1mol-1 = 122.0 J K-1mol-1
c) Br2 (l) ® Br2 (g)DS° = 245.46 J K-1mol-1 - 152.2 J K-1mol-1 = 93.26 J K-1mol-1
17) a) ½ I2 (s) + ½ Cl2 (g) ® ICl (g)
DS° = 247.55 J K-1mol-1 - [0.5(116.14 J K-1mol-1) + 0.5(223.07 J K-1mol-1)] = 77.95 J K-1mol-1
b) C (s, graphite) + ½ O2 (g) + Cl2 (g) ® COCl2 (g)DS° = 283.53 J K-1mol-1 - [5.74 J K-1mol-1 + 0.5(205.14 J K-1mol-1) + 223.07 J K-1mol-1]
= -47.85 J K-1mol-1c) Ca (s) + C (s, graphite) + 1½ O2 (g) ® CaCO3 (s)
DS° = 92.9 J K-1mol-1 - [41.42 J K-1mol-1 + 5.74 J K-1mol-1 + 1.5(205.14 J K-1mol-1)]
= -262.0 J K-1mol-1
21) H2O (l) ® H2(g) + ½ O2 (g)
This reaction should have a positive DS as well as a positive DH (since it is highly endothermic). These values can be quantified as follows:
DS° = [130.68 J K-1mol-1 + 0.5(205.14 J K-1mol-1)] - 69.96 J K-1mol-1 = +163.29 J K-1mol-1
DH° = +285.8 kJ mol-1
Consequently, this reaction is expected to be spontaneous only at high
temperature (~ 1500ºC).
25) a) Mg (s) + 2 H2O (l)
® Mg(OH)2 (s) + H2
(g)
DSsystem = [63.18 J K-1mol-1 + 130.68 J K-1mol-1] - [32.68 J K-1mol-1 + 2(69.91 J K-1 mol-1)]= + 21.36 J K-1mol-1
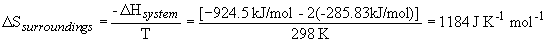
DSuniverse = DSsystem + DSsurroundings = 1205 J K-1mol-1
29) In the following problems, T = 298 K.
a) Mg (s) + O2 (g) + H2 (g) ® Mg(OH)2 (s)DHºf = -924.54 kJ/molDSº = 63.18 J K-1mol-1 - [32.68 J K-1mol-1 + 130.68 J K-1mol-1 + 205.14 J K-1mol-1]
= -305.32 J K-1mol-1
DGºf = DHºf - TDS = -833.55 kJ/mol
b) ½ N2 (g) + ½ O2 (g) + ½ Cl2 (g) ® NOCl (g)DHºf = + 51.71 kJ/molDSº = 261.8 J K-1mol-1 - [0.5(191.6 J K-1mol-1) + 0.5(223.07 J K-1mol-1) +
0.5(205.14 J K-1mol-1)] = -48.11 J K-1mol-1
DGºf = DHºf - TDS = + 66.05 kJ/mol
c) 2 Na (s) + C (s, graphite) + 1½ O2 (g) ® Na2CO3 (s)DHºf = -1130.68 kJ/molDSº = 134.98 J K-1mol-1 - [2(51.21 J K-1mol-1) + 5.74 J K-1mol-1 + 1.5(205.14 J K-1mol-1)]
= -280.89 J K-1mol-1
DGºf = DHºf - TDS = -1,047 kJ/mol
33) a) HgS (s) + O2 (g) ®
Hg (l) + SO2 (g)
DG° rxn = [DG° f (Hg) + DG° f (SO2)] - [DG° f (HgS)+ DG° f (O2)]DG° rxn = [0 kJ/mol + (-300.19 kJ/mol)] - [(-50.6 kJ/mol)+ 0 kJ/mol] = -249.6 kJ/mol
b) 2 H2S (g) + 3 O2 (g) ® 2 H2O (g) + 2 SO2 (g)
DG° rxn = [2 DG° f (H2O) + 2 DG° f (SO2)] - [2 DG° f (H2S)+ 3 DG° f (O2)]
DG° rxn = [2(-228.57 kJ/mol) + 2(-300.19 kJ/mol)] - [2(-33.56 kJ/mol)+ 3(0 kJ/mol)]
= -990.38 kJ/mol
c) SiCl4 (g) + 2 Mg (s) ® 2 MgCl2 (s) + Si (s)
35) TiCl2 (s) + Cl2 (g) ® TiCl4 (l) DGºrxn = -272.8 kJ/mol
DG° rxn = [2 DG° f (MgCl2) + DG° f (Si)] - [DG° f (SiCl4)+ 2 DG° f (Mg)]
DG° rxn = [2(-591.79 kJ/mol) + 0 kJ/mol] - [-616.98 kJ/mol + 2(0 kJ/mol)]
= -566.6 kJ/mol
DG° rxn = [DG° f (TiCl4)] - [DG° f (TiCl2)+ DG° f (Cl2)]-272.8 kJ/mol = [-737.2 kJ/mol] - [DG° f (TiCl2) + 0 kJ/mol]
DG° f (TiCl2) = -464.4 kJ/mol
39) C (s, graphite) + ½ O2 (g) + 2 H2
(g) ® CH3OH (l)
DG° = -RT ln K
43) a) 2 C (s, graphite) + H2 (g) ® C2H2 (g)
DS° = 200.94 J K-1mol-1 - [2(5.74 J K-1mol-1) + 130.68 J K-1mol-1] = 58.78 J K-1mol-1
b) 2 C (s, graphite) + 2 H2 (g) ® C2H4 (g)49) 2 Ag2O (s) ® 4 Ag (s) + O2 (g)DS° = 219.56 J K-1mol-1 - [2(5.74 J K-1mol-1) + 2(130.68 J K-1mol-1)] = -53.28 J K-1mol-1
c) 2 C (s, graphite) + 3 H2 (g) ® C2H6 (g)DS° = 229.6 J K-1mol-1 - [2(5.74 J K-1mol-1) + 3(130.68 J K-1mol-1)] = -173.92 J K-1mol-1
DH° rxn = [4 DH° f (Ag) + DH° f (O2)] - [2 DH° f (Ag2O)] = + 62.10 kJ/molDS° rxn = [4 S° (Ag) + S° (O2)] - [2 S° (Ag2O)] = + 132.7 J K-1 mol-1
Since both the changes in enthalpy and the change in entropy for this reaction are positive, the reaction will become spontaneous (or, "product-favored") at high temperature. At 25° C, we have,
DG° rxn = DH° rxn - T DS° rxn = + 22.45 kJ/mol (not spontaneous)
To find out where the reaction becomes spontaneous, we set DG° rxn (the equilibrium condition) and solve for T. We make the assumption that DH° rxn and DS° rxn are independent of temperature, which is a good approximation in most cases.
53) CH3OH (l) ®
CH4 (g) + ½ O2 (g)
a) DS° rxn = [S° (CH4) + ½ S° (O2)] - [S° (CH3OH)] = + 162.03 J K-1 mol-1The positive entropy change is expected, since a liquid usually has lower entropy than a gas.
b) DH° rxn = [DH° f (CH4) + ½ DH° f (O2)] - [DH° f (CH3OH)] = + 163.85 kJ/mol
DG° rxn = DH° rxn - T DS° rxn = + 115.6 kJ/mol (not spontaneous)
c) The temperature at which the reaction becomes spontaneous is:
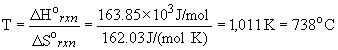
59) a) H2O (l) ®
H2 (g) + O2 (g)
DH° rxn: positive DS° rxn: positive DG° rxn: positive
b) explosion of dynamite:61) The reactions are:DH° rxn: negative DS° rxn: positive DG° rxn: negative
c) combustion of gasoline:
DH° rxn: negative DS° rxn: positive DG° rxn: negative
Reaction 1: AgCl (s) « Ag+ (aq) + Cl- (aq) K1 = Ksp = 1.8 ´ 10-10Reaction 2: Ag+ (aq) + 2 Cl- (aq) « AgCl2- (aq) K2 = Kf = 2.5 ´ 105
Sum: AgCl (s) + Cl- (aq) « AgCl2- (aq) Ksum = K1K2 = 4.5´ 10-5
The free energy changes for the above reactions are:
DG° rxn1 = [DG° f (Ag+) + DG° f (Cl-)] - [DG° f (AgCl)] =
= [77.1 kJ/mol + (-131.2 kJ/mol)] - [-109.79 kJ/mol] = +55.7 kJ/mol
DG° rxn2 = [DG° f (AgCl2-)] - [DG° f (Ag+) + 2 DG° f (Cl-)] =
= [-215.4 kJ/mol] - [77.1 kJ/mol + 2(-131.2 kJ/mol)] = -30.1 kJ/mol
DG° sum = [DG° f (AgCl2-)] - [DG° f (AgCl) + DG° f (Cl-)] == [-215.4 kJ/mol] - [-109.79 + (-131.2 kJ/mol)] = +25.59 kJ/mol
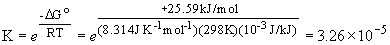
This result is similar to that obtained using the equilibrium constants of the two "component" reactions.
64) C6H6 (l) + H2O (l)
® C6H5OH (s)
+ H2 (g)
DS° = [S° (C6H5OH) + S° (H2)] - [S° (C6H6) + S° (H2O)] = + 162.03 J K-1 mol-1DS° = [144.0 J K-1 mol-1 + 130.68 J K-1 mol-1] - [69.91 J K-1 mol-1 + 172.8 J K-1 mol-1]
= + 31.97 J K-1 mol-1DH° rxn = [DH° f (C6H5OH) + DH° f (H2)] - [DH° f (C6H6) + DH° f (H2O)]
DH° rxn = [-165.1 kJ/mol + 0 kJ/mol] - [49.03 kJ/mol + (-237.13 kJ/mol)] = +23.0 kJ/mol
DG° rxn = DH° rxn - T DS° rxn = + 13.5 kJ/molThis reaction is not spontaneous (i.e., it is reactant favored) at standard temperature. It is an entropy-driven process.
b) The oxygen atom is sp3 hybridized. Therefore, the ideal VSEPR bond angle for the C-O-H- bond would be 109.5° .c) The hybridization of the carbon atoms in phenol is sp2.
d) This is a typical weak acid problem. The reaction is:
C6H5OH (aq) + H2O (l) ® C6H5O- (aq) + H3O+ (aq)
C6H5OH (M) C6H5O- (M) H3O+ (M) Initial 0.012 0 0 D -x x x Final 0.012-x x x