Chemistry Homework #1
Chapter 13 - Chemical Kinetics
3.
![]()
5. From the information provided, we can state:
rate µ [H2]rate µ [NO]2
So, rate = k [H2] [NO]2
b) Using x as the reference concentration of H2, and y as the reference concentration of NO, we can say,rate1 = k xy2
If the concentration of H2 is doubled and the concentration NO is tripled, we can substitute into the above expression to yield,
rate2 = k (2x)(3y)2 = 18 k xy2
So, the rate observed with the increased concentrations is 18 times greater than that seen under the initial conditions.
7. The form of the rate expression is: rate = k [CH3I]m
[C5H5N]n.
Comparing experiments two and three reveals that, while keeping C5H5N constant, an increase in the CH3I concentration by a factor of two results in a rate increase of a factor of two. That is, rate µ [CH3I]1. This can also be shown by:
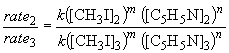
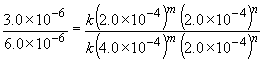
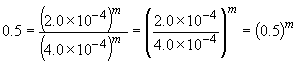
log 0.5 = m log 0.5
m = 1
Since the value of m is known, a similar approach can be used to determine the value of n by comparing the rate and concentration data for experiments 1 and 2.
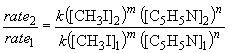
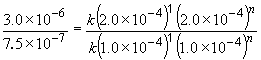
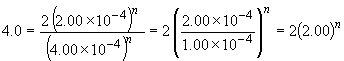
log 2.0 = n log 2.0
n = 1
Therefore, the rate law is: rate = k [CH3I] [C5H5N].
b) The rate constant is given by (using data from experiment 1)
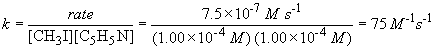
The same value of k would be obtained using the data from the second or third data sets as well.
c) The rate of the reaction for the conditions listed is given by the rate law found in (a) and the rate constant found in (b).
rate = k [CH3I] [C5H5N]
rate = (75 M-1 s-1) (2.0 ´ 10-5 M) (5.0 ´ 10-5 M) = 7.5 ´ 10-8M s-1
9. Using the first order integrated rate law:
13. This problem also requires the use of the first order integrated rate law: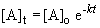
Converting the partial pressure of SO2Cl2 to concentration (which, by the way, is not necessary in this problem, but is a good habit when dealing with gas problems) gives:
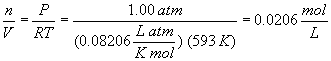
The concentration when the partial pressure is 0.5 atm is 0.0103 M.
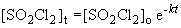
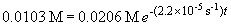
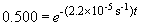
ln 0.500 = -t (2.2 ´ 10-5 s-1)
t = 31,500 s = 8.75 hours
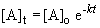
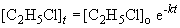
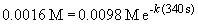
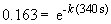
ln 0.163 = -k (340 s)
k = 0.0053 s-1
15. This problem requires use of the second order integrated rate
law. The reaction is:
2 I ® I2
Setting c equal to 1/[I]o yields the correct form of the integrated rate law
Note that the form of the integrated rate law depends on the stoichiometry of the balanced equation.
Now, solving the question….
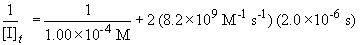
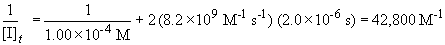
[I]t = 2.34 ´ 10-5 M
17. This problem also requires the integrated second order rate
law, but it will have a slightly different form than that used above. This
is because in the above problem, the reaction was second order in I. In
this problem, even though it is second order overall, it is first order
in the reactant of interest. So, beginning with the derivation of the rate
law, we have:
OH- (aq) + NH4+ (aq) ® H2O (l) + NH3 (aq)
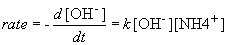
But, in this reaction, the concentration of [OH-] = [NH4+], so we can simplify the differential equation by performing a simple substitution:
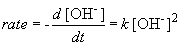
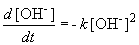
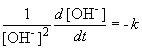
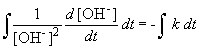
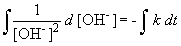
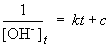
Setting c equal to 1/[OH-]o yields the correct form of the integrated rate law:
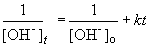
Now, solving the question….
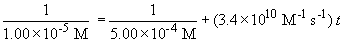
t = 2.88 ´ 10-6 sec
47. The reaction is:
Hb (aq) + O2 (aq) ® HbO2 (aq) (Hb = hemoglobin)
The rate law is: rate = k [Hb] [O2]k = 4 ´ 107 M-1 s-1
rate = (4 ´ 107 M-1 s-1) (2 ´ 10-9 M) (5 ´ 10-5 M) = 4.0 ´ 10-6 M s-1
49. You need to be careful with the integrated rate law in this
problem. To illustrate, let's re-derive the integrated expression for this
system. The balanced equation is:
3 In+ (aq) ® 2 In (s) + In3+ (aq)
Since we are told the reaction is first order, the rate can be expressed as:
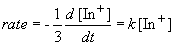
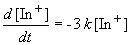
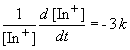
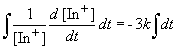
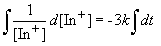
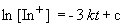
Setting c equal to [In+]o yields the correct form of the integrated rate law:
[In+]t = [In+]oe-3kt
From the above equation, it is apparent that the slope of ln[In+] vs time will have a slope of -3k. The plot is shown below, and the resulting rate constant is 3.37 ´ 10-4 s-1.
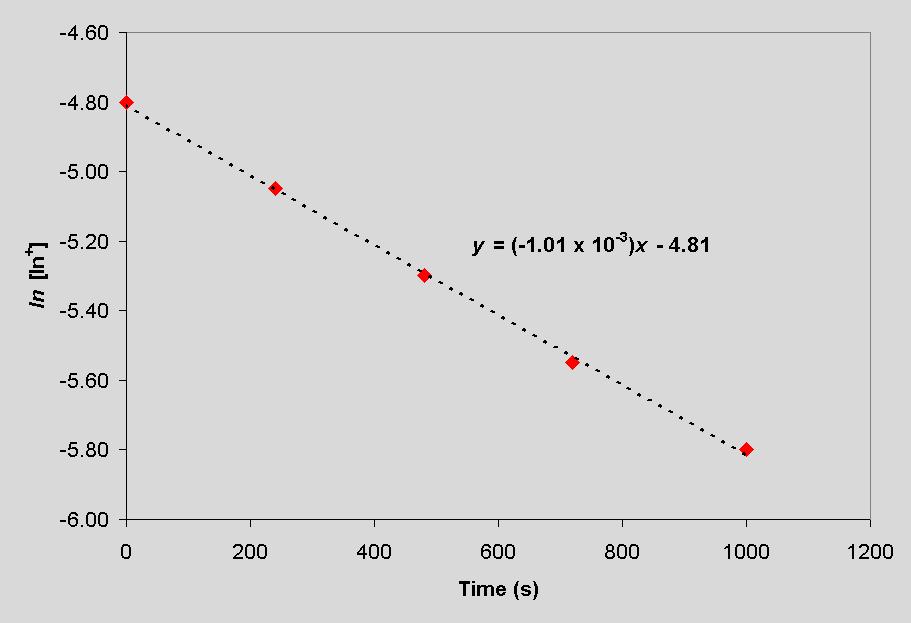
The half-life of the reaction, t1/2, is the time at which the concentration of In+ is equal to one-half the initial concentration. Or, [In+]t1/2 = 0.5 [In+]o. Substituting into the above expression yields,
0.5 [In+]o = [In+]o e-3kt1/2
0.5 = e-3kt1/2
ln (0.5) = -3kt1/2
69. The enthalpy change, DH, for the
reaction is: +52.96 kJ
The rate law is:rate = k [H2] [I2]
rate = (0.0242 M-1 s-1) (0.081 M) (0.036 M) = 7.1 ´ 10-5 M s-1.
Using the definition of rate, we can say,
70. Given the rate law, rate = k [ClO2]2[OH-],
we need to know [OH-] if we are to find the initial rate of
the reaction. Since the concentration of CN- and HCN are provided
(a conjugate acid-base pair….ding! ding! ding! ding! - a Buffer!), the
Henderson-Hasselbach equation can be used to find H3O+,
and then OH-.
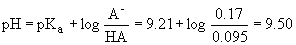
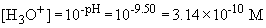
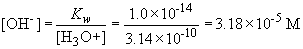
Now, the rate can be calculated:
rate = k [ClO2]2[OH-]
rate = (230 M-2 s-1) (0.020 M)2 (3.2 ´ 10-5 M) = 2.9 ´ 10-6 M s-1
71. Given the total pressure and the mole fractions, the partial
pressures of NO and O3 are:
PNO = (0.00057) (3.26 atm) = 1.85 ´ 10-3 atmPO3 = (0.00026) (3.26 atm) = 8.48 ´ 10-4 atm
From these partial pressure, the corresponding concentrations are:
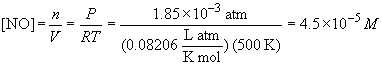
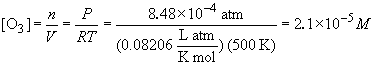
The rate law is:
rate = k [NO] [O3] = (7.6 ´ 107 M-1 s-1) (4.5 ´ 10-5 M) (2.1 ´ 10-5 M)
rate = 7.1 ´ 10-2 M s-1