BrO (g) + NO (g) ® Br (g) + NO2 (g)
kf = 1.3 ´ 1010 M s-1
![]()
Spring Chemistry Homework #2
Chapter 13 - Chemical Kinetics
21a)
H2O2® H2O + O (unimolecular)
ClO + O3 ® Cl + 2 O2 (bimolecular)
Cl + CF2Cl ® CF2Cl2 (bimolecular)
b) The overall reaction is: H2O2 + O3® H2O + 2 O2
c) The reaction intermediates are: O, ClO, CF2Cl and Cl.
CF2Cl2 is not
an intermediate, but a catalyst for the reaction.
BrO (g) + NO (g) ® Br (g) + NO2 (g)
kf = 1.3 ´ 1010 M s-1
![]()
At equilibrium, the rate of the forward reaction will be equal to that of the reverse reaction. So, we can say,rateforward = ratereverse
Since this is an elementary reaction, we know the rate laws and can say:
kf [BrO] [NO] = kr [Br] [NO2]
Or,

27a) The rate law will be:
Since H is an intermediate formed in a rapid pre-equilibrium, we can write:
![]()
or,
![]()
This is inconsistent with the observed kinetic data, so mechanism "a"
can be eliminated as a potential reaction pathway.
There are two pre-equilibria in this mechanism, so:
![]()
![]()
So rate law is:
![]()
This is the observed rate law, so mechanism "b" is consistent with the
observed rate data.
There are two pre-equilibria in this mechanism, so:
![]()
![]()
So the rate law is:
![]()
This is inconsistent with the observed rate law, so mechanism "c" can
be ruled out as a possible reaction pathway.
37. Using the Arrhenius equation at two temperatures gives: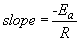
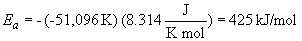
The pre-exponential factor is obtained from the intercept of the plot:
y-intercept = ln A
41. For the reaction: OH (g) + HCl (g) ® H2O (g) + Cl (g),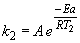
Dividing the first equation by the second yields:
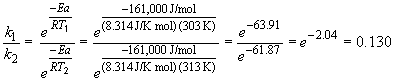
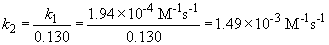
b) The integrated rate law for this reaction is:
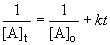
Since, k and t are known at 30.0° C, we can write:
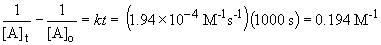
So, at 40.0° C, the time required to consume the equivalent amount of reactant is:
0.194 M = kt
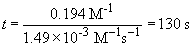
the difference in energy between the reactants and the transition state, which is the activation energy, is + 3.5 kJ/mol.51. The deprotonation reaction of hydrogen cyanide is:DE for the reaction is -66.8 kJ/mol. Thus, the difference in energy between the transition state and the products is: +70.3 kJ/mol. This is equivalent to the activation energy of the reverse reaction, H2O (g) + Cl (g) ® OH (g) + HCl (g).
HCN (aq) + OH- (aq) « CN- (aq) + H2O (l)
59. The mechanism is:
CH3CHO ® CH3 + CHO (initiation)CH3 + CH3CHO ® CH4 + CH2CHO (propagation)
CH2CHO ® CO + CH3 (propagation)
CH3 + CH3 ® CH3CH3 (termination)
In this mechanism, CH3 and CH2CHO are reactive intermediates that are consumed and generated by the propagation steps - this is the defining characteristic of chain reactions. In this case, the product of the second reaction reacts in a third reaction to regenerate the reactant of the second reaction, thereby allowing the steps to be repeated. This results in the "chain". The other products of the propagation steps, CH4 and CO, are the main products of the reaction. The termination step is the reaction of two reactive intermediates to form an inert product (which is a minor product and does not appear in the balanced equation).
61. This is an Arrhenius activation energy problem. We can begin
by making some prelimary assumptions. Let's say the the term "fully cooked"
corresponds to the following reaction being 99.99% complete:
Foodraw ® Foodcooked
Furthermore, let's assume that the kinetics of the cooking reaction follows first order kinetics (the rate at which eggs boil depends linearly on the number of eggs being cooked!). So, we can calculate the rate constant by knowing that the reaction is "complete" in 5 minutes at 112 C.So,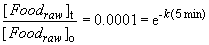
ln (0.0001) = -k (5 min)
k = 1.84 min-1
At 100° C, the reaction takes 10 minutes to be complete, so the rate constant is:Now, the Arrhenius equation can be used to calculate the activation energy of the cooking reaction: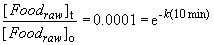
ln (0.0001) = -k (10 min)
k = 0.92 min-1
b) To calculate the time it would take to "fully cook" the sample in Denver, the Arrhenius equation is again used. First calculate k, then use the new rate constant in the integrated rate law.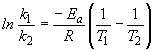
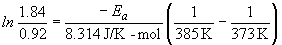
Ea = 70 kJ/mol
Thus, it takes longer to cook food at higher elevation (lower temperatures). Just like you knew it would.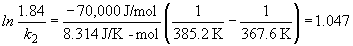
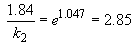
k2 = 0.646
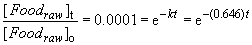
ln (0.0001) = -(0.646 min-1) t
t = 14.25 min.