![]()
Spring Chemistry Homework #3 Answer Key
Introduction to Quantum Chemistry
Oxtoby, Nachtrieb and Gillis: Chapter 15:
5.
11. The work function is the amount of energy that a single photon must carry to eject a photon from the surface of a metal. If the work function for cesium is 3.43 × 10-19 J, then,
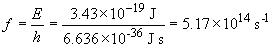
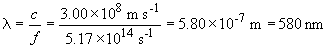
This wavelength corresponds to yellow light. So wavelengths of light that have a wavelength of 580 nm or less will result in a photocurrent from cesium. This includes green, blue and violet light, but not orange or red.
For selenium, the work function is 9.5 × 10-19 J. This requires light in the ultra-violet portion of the spectrum to produce a photocurrent.
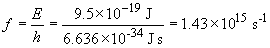
25. The radius of "hydrogen-like" atoms is given by:
31. The de Broglie wavelength of a particle is given by: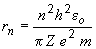
where:
n: Bohr quantum level
eo: permitivity of free space
Z: atomic number (magnitude of nuclear charge)
e: electron charge
m: mass of the electronFor an electron in the third quantum level of a B4+ ion, we have:
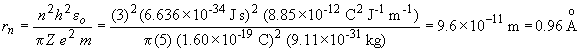
The energy of a hydrogen-like atom is given by:
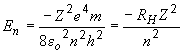
where RH is the Rydberg constant (2.18 × 10-18 J). So, for the B4+ ion with an electron in the n = 3 quantum level:
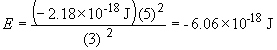
For an n = 3 to n = 2, we have DE = E2 - E3. Or,
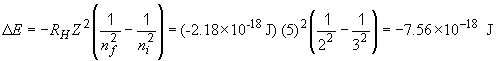
This is the energy change for the atom itself - it is negative because the atom went from an excited, or "high energy", state to a lower energy state. The energy "lost" was emitted as a photon with the following frequency:
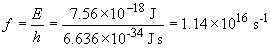
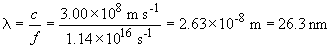
To remove an electron a B4+ ion in the n = 3 state would require:
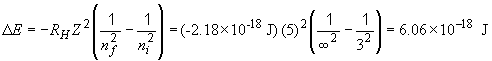
So, for 1 mol of such ions,
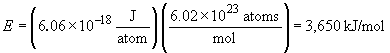
72. Green light has a wavelength around 500 nm. This corresponds to a photon with the following energy: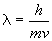
a) For an electron moving at 1.00 × 103 m s-1:
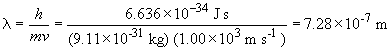
b) For a proton moving at the same speed:
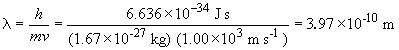
c) For a baseball thrown by Sidd Finch,
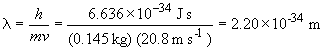
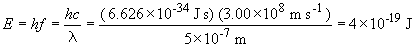
To find a possible transition of the C5+ ion that would a photon of this energy, we can do the following:
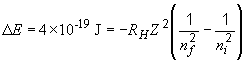
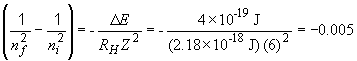
The value 0.005 is close to 0.00478, which corresponds to the term on the left, when nf = 7 and ni = 8.
The actual wavelength for an n = 8 to n = 7 transition in the C5+ ion has a wavelength of 530 nm, which is still in the green portion of the spectrum.
90. The energy required to raise the water temperature is:
DeKock & Gray: Chapter 1: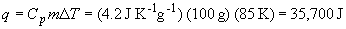
The energy of one photon of the given frequency is:
E = hf = (6.636 × 10-34 J s) (2.45 × 109 s-1) = 1.63 × 10-24 J
So, the total number of photons required is:

2. a) The n = 1 has the ground state configuration.
b) The electron is moving faster
in the n = 1 state.
c) The n = 4 state has
the larger radius.
d) The n = 1 state has
the lower potential energy
e) The n = 1 state has
the larger ionization energy.
5.
6.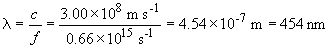
E = hf = (6.636 × 10-34 J s) (0.66 × 1015 s-1) = 4.38 × 10-19 J
Wavenumbers are a means of expressing frequency. It corresponds to the number of cyclces per centimeter, or, cm-1.
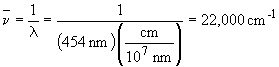
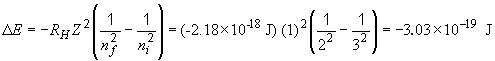
So the energy released is + 3.03 × 10-19 J.
18. Three lines are observed because there are three transitions
possible. These are:
n = 3 to n = 2
n = 3 to n = 1n = 2 to n = 1
The shortest wavelength of light corresponds to the n = 3 to n = 1. This has a frequency of :
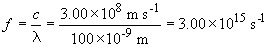
The energy difference between the first and third levels is:
E = hf = (3.00 × 1015 s-1) (6.626 × 10-34 J s) = 1.99 × 10-18 J
For the 125 nm light:
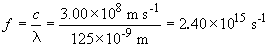
The energy difference between these levels is:
E = hf = (2.40 × 1015 s-1) (6.626 × 10-34 J s) = 1.59 × 10-18 J
For the 500 nm wavelength light:
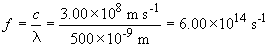
The energy difference between these levels is:
E = hf = (6.00 × 1014 s-1) (6.626 × 10-34 J s) = 3.98 × 10-19 J