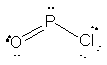
Chemistry Homework #7
Oxtoby, Nachtrieb & Gillis, Chapter 3: Classical Bonding
61. The Lewis structure for OPCl is shown below
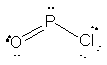
Two resonance forms for O2PCl are shown below. The structure
to the left has zero formal charges on all atoms (and so is favored energetically),
while the structure on the right satisfies the octet for all atoms.
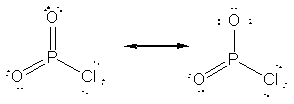
64. I may be missing something, but I could only come up with three equivalent resonance structures for S3N3-. Three additional resonance forms that are equivalent to each other, but not the top three, are also shown. The top three forms have a formal charge on one nitrogen each; all other atoms have zero formala charges. The bottom three structures have more formal charges (-1 on two of the nitrogen, +1 on one sulfur) and hence would not be predicted to be as important contributors to the resonance hybrid.
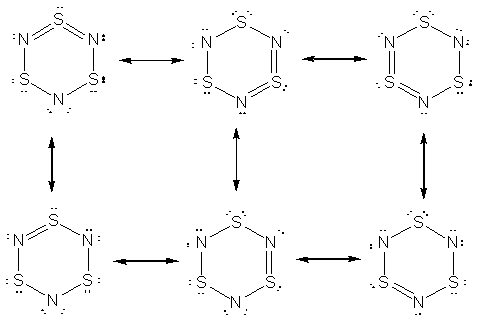
The average formal charge of the nitrogen atoms in the first three resonance forms is -1/3; on the sulfur atoms it is zero. In the second set of resonance forms (bottom), the average formal charge on the nitrogen is -2/3, while that on the sulfur is +1/3.
The advanced calculation, confirms the above analysis. The most important resonance forms are the top three. The calculated charges are close to the -1/3 and 0 for the nitrogen and sulfur atoms, respectively. The deviations arise from the fact that the second set of resonance forms is not expected to contribute much to the actual structure, but they do have a minor effect, making the nitrogen slightly more negative and the sulfurs slightly more positive than the most stable resonance forms predict.
The charges of -0.375 for nitrogen and +0.041 for sulfur are consistent
with the overall charge on S3N3- since
sum of the formal charges on all atoms must equal the overall charge on
the species. So, 3(-0.375) + 3(+0.041) = -1.002 (or -1).
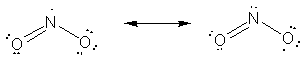
65. This was an interesting problem (to me) because the result is counterintuitive. The molecule NO2 dimerizes to form N2O4, which has a nitrogen-nitrogen bond (see below). This implies that the unpaired electron involved in bond formation is on the nitrogen. However, when considering the two resonance forms below, the form on the right has zero formal charges while the structure with the unpaired electron on the nitrogen has a +1 charge on the nitrogen and a -1 formal charge on one of the terminal oxygen atoms. So, the electroneutrality principle seems to give a prediction at odds with the observed chemical behavior of the compound. To gain greater insight into the bonding of NO2 would require the use of more sophisticated models (i.e., molecular orbital theory) rather than simple classical approaches.
74. A virtual menagerie of xenon critters.
|
|
state |
on Xenon |
|
|
geometry |
|
|
|
|
|
|
|
|
|
|
|
|
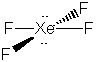 |
|
|
|
|
|
|
 |
|
|
|
|
|
|
 |
|
|
|
|
|
|
 |
|
|
|
|
|
|
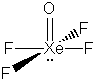 |
|
* Lone pairs on terminal atoms not shown; all have complete octets
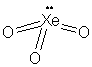
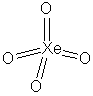
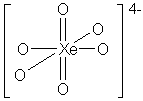
H4XeO6 is easier
to consider as the deprotonated conjugate base, XeO64-;
the H+ ions would be located on the singly bonded terminal oxygen
atoms of the structure; protonation would not significantly affect the
xenon geometry.
78.
| compound |
|
|
|
| C2H6 |
 |
|
|
| C2H4 |
 |
|
|
| C2H2
|
|
|
|
| C2 |
|
|
|
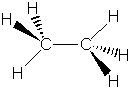
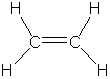
As can be seen from these data, there is a strong correlation
between bond order and bond length for C2H6, C2H4
and C2H2. As the bond order increases, the bond length
decreases, as would be expected for a stronger force pulling two nuclei
together.
The molecule C2 is problematic however. The
bond length is close to that of the doubly bonded C2H4.
However, for carbon to complete its octet, a quadruple bond must exist
between the two atoms. Which to believe? Well, the data don't lie and theory
should reflect and explain physical observation. The problem in this case
is a limitation of classical bonding concepts to explain bonding in a molecule
such as C2. As it turns out, molecular orbital theory (is your
appetite whetted yet?) predicts a bond order of 2 for this species, a value
that is in good agreement with the observed properties of the molecule.
85. An obnoxious question, I know just keeping you on your toes. To get the empirical formula, assume you have 1.000 g of the compound and proceed as follows:
![]()
![]()
![]()
So, the molar ratio can be expressed as: Cl0.009763O0.02928F0.009759. Dividing through by the smallest term yields, ClO3F.
The least electronegative element is chlorine, so this is expected to be the central atom of the molecule. So, the most stable resonance form is:
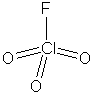
Other resonance form can also be written, but this one
has zero formal charges on all atoms (lone pairs on terminal atoms have
been omitted; all have complete octets). Note that the chlorine has a +7
oxidation state, so its use as a rocket fuel is certainly plausible. The
chlorine is expected to have tetrahedral geometry.
86. The three permutations of N, S and F are shown below. The structure on the right is awful - fluorine has a positive formal charge and sulfur has a negative one. In addition, fluorine is the central atom and in only a few cases does fluorine bridge two other atoms.
![]()
![]()
![]()
The first two structures are not bad. In each case, the
formal charges on all atoms is zero. The first structure, which is the
compound that is observed in nature, has sulfur occupying the central position.
There is a tendency, and this is just one example, that central atoms tend
to be the least electronegative in a given molecule. This can be understood
by the fact that elements with low electronegativities do not hold electron
pairs as tightly and, therefore, they are more readily available for bond
formation.
DeKock & Gray - Chapter 2 -Atomic & Molecular Properties
16. The Lewis structures for NF3, PF3 and PF5 are shown below.
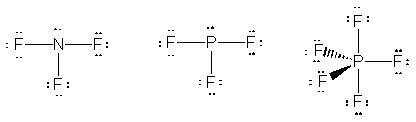
The existence of PF5 is attributed to promotion of electrons in phosphorous into vacant 3d orbitals. That is all five valance electrons can be unpaired and made available for bonding. The promotion of the electron can be written as:
[Ne] 3s2 3px1 3py1 3pz1 ® [Ne] 3s1 3px1 3py1 3pz1 3d1
Nitrogen, because it has no available d orbitals in its valence
shell, cannot promote an electron into another orbital. In effect, the
two 2s electrons are "locked away" and are unavailable for bonding. Based
on this argument, elements in the third row of the periodic table, the
first level in which element have d orbitals in their valence shells,
should be able to expand their octets. So, of the compounds listed SF2,
SF4 and SF6 should all exist (and they do, although
SF2 is not as stable as its Lewis structure implies), while
OF4 and OF6 should not (and they don't). The only
oxygen-fluorine compound listed that does exist is OF2 because
that does not violet the octet for oxygen.
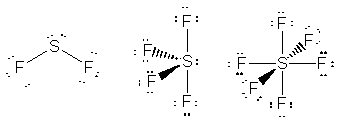
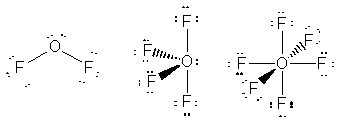
exists non-existent non-existent
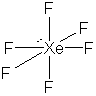
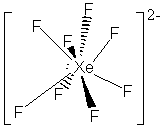
23. Another virtual menagerie of xenon beasties.
|
|
state |
on Xenon |
|
|
geometry |
|
|
|
|
|
|
|
|
|
|
|
|
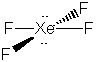 |
|
|
|
|
|
|
 |
|
|
|
|
|
|
 |
|
|
|
|
|
|
 |
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
* Lone pairs on terminal atoms not shown; all have complete octets
The formal charges on all of the atoms in these structures is zero except: Xe = -2 in XeF82-, Xe = +1 is XeF+.
The Xe-F bond in XeF4 is expected to be shorter
than the I-F bond in IF4-. This is because in the
neutral atoms, Xe is smaller than I. Also, the formal charge on I in IF4-
is -1, compared to 0 for Xe in XeF4; there will therefore be
additional electron-electron repulsion around I-; this will
tend to enlarge the central atom beyond what would be expected for a neutral
species.
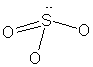
33/34.
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
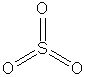 |
|
|
|
|
|
|
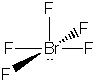 |
|
|
|
|
|
|
|
|
(but it is an ion) |
|
|
|
|
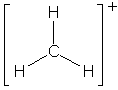 |
|
|
|
|
|
|
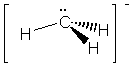 |
|
|
|
|
|
|
 |
|
|
* Lone pairs on terminal atoms not shown; all have complete octets
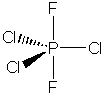
For PCl3F2, an
isomer can be drawn in which the fluorine atoms occupy equatorial positions;
this isomer would be polar.