![]()
![]()
Therefore the empirical formula is C2H6O.
Review Assignment #3
Chapter 3: Stoichiometry
46.
a. find mole ratios relative to oxygen:
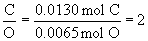
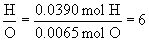
Therefore the empirical formula is C2H6O.
b.
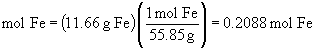
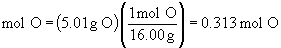
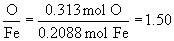
So, the empirical formula is FeO1.5, (yuck), or preferably, Fe2O3.
c. For problems that provide mass percentages, assume you start
with a mass of 100g.
Therefore the empirical formula is CH2O.
52. a)
60. The balanced equation is: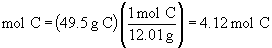
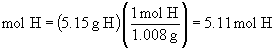
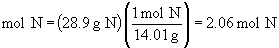
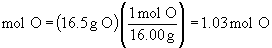
Find ratios relative to oxygen:
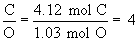
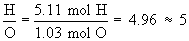
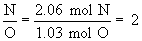
Therefore the empirical formula is C4H5N2O.
The empirical formula weight is 97.1. So the number of formula units per molecule is given by:
Therefore, there are two "C4H5NO2 units" per molecule of caffeine, so its molecular formula is C8H10N4O2.
b)
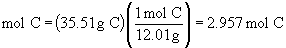
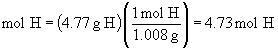
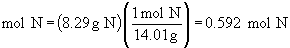
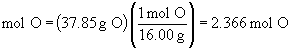
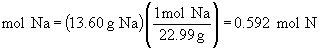
Find ratios relative to sodium:
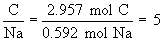
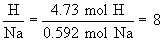
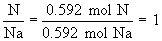
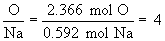
Therefore the empirical formula is NaC5H8NO4.
The empirical formula weight is 169.1. Since this is equivalent to the molar mass, the empirical formula and the molecular formulas are the same in this case.
Na2SiO3 (s) + 8 HF (aq) ® H2SiF6 (aq) + 2 NaF (aq) + 3 H2O (l)
a)
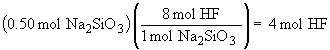
b)
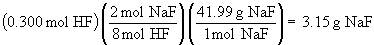
c)
66. The balanced equation is:
4 C3H5O9N3 (s) ® 12 CO2 (g) + 6 N2 (g) + O2 (g) + 10 H2O (g)
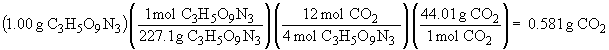
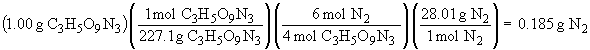
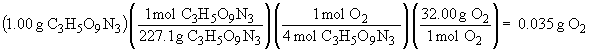
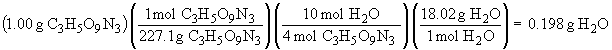
The sum of all the masses of the products is: 0.999 g. The small deviation from 1.00 is due to rounding errors in the calculations, but the result is in accord with The Law of Conservation of Mass.
74. The balanced equation is:
4 NH3 (g) + 5 O2 (g) ® 4 NO (g) + 6 H2O (g)
To determine which is the limiting reactant, calculate the mass of product possible based on the masses of each reactant individually.
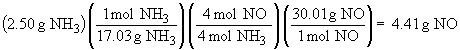
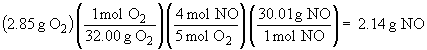
Since complete reaction of O2 yields a smaller amount of product than does complete reaction of NH3, the O2 is the limiting reactant. Only 2.14 g of NO form.
The amount of excess NH3 can be found by calculating the amount of NH3 consumed and then finding the difference from the original mass:
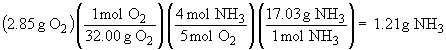
NH3 remaining: 2.50 g - 1.21 g = 1.29 g NH3.
78. The balanced equation for this reaction is:
Na2CO3 (aq) + 2 AgNO3 (aq) ® Ag2CO3 (s) + 2 NaNO3 (aq)
This is a limiting reactant problem.
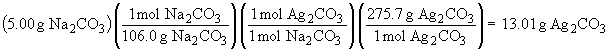
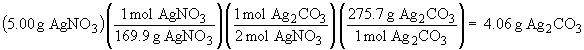
Thus, AgNO3 is the limiting reactant and will be completely consumed. 4.06 g of Ag2CO3 will be formed. The amount of NaNO3 that forms is:
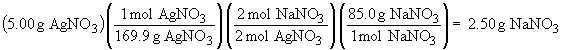
The amount of Na2CO3 that reacts is:
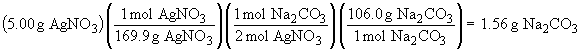
Therefore, the amount of Na2CO3 that remains when the reaction is complete is: 5.00 g - 1.56 g = 3.44 g. So, in summary, the masses of the salts are:
| silver nitrate | 0.00 g |
| sodium carbonate | 3.44 g |
| silver carbonate | 4.06 g |
| sodium nitrate | 2.50 g |
90. This is a challenging problem that requires you to apply the mole concept in a creative way. The balanced reaction is:
2 XI3 + 3 Cl2 ® 2 XCl3 + 3 I2
From the stoichiometry, we know that the number of moles of XI3 is equal to the number of moles of XCl3. We also know the masses that correspond to each of these materials. But, we do not know the actual number of moles because we don't know the molar mass. We can, however, express the molar masses the following way:molar mass XI3 = [x + 3(126.9)] g/mol
molar mass XCl3 = [x + 3(35.45)] g/mol
We can then write a stoichiometric relationship in the usual way:
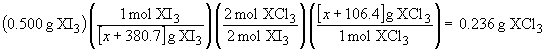
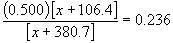
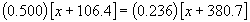
0.500 x + 53.2 = 0.236 x + 89.84
0.264 x = 36.64
x = 138.8
This is the atomic weight of element X; X is therefore Lanthanum!
97. The balanced chemical reaction is:
C57H110O6 + 163/2 O2 ® 57 CO2 + 55 H2O
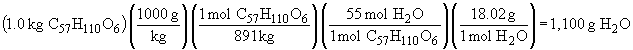
107. There are two reactions at work here:
S (s) + O2 (g) ® SO2 (g)
SO2 (g) + CaO (s) ® CaSO3 (s)
The mass of sulfur burned each day is: (2000 tons) (0.025) = 50 tons.Chapter 10: GasesSetting up the stoichiometric relationships yields:
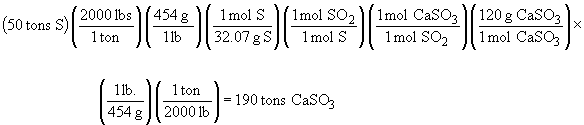
10 a)
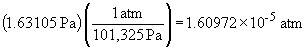
b)
18. a) This is an example of Boyle's Law:
P1V1 = P2V224.
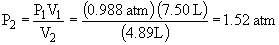
b) This is an example of Charles' Law:
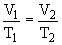
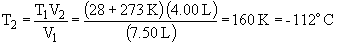
28.
a)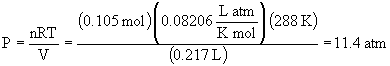
b)
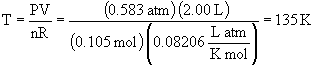
c)
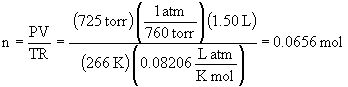
d)
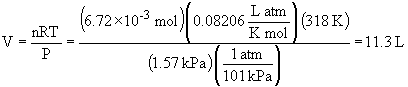
30. a)
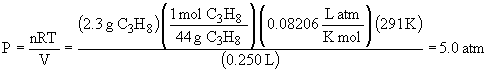
b) STP = 1.00 atm, 273 K.
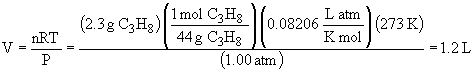
c) 130° F = 54° C = 327 K
41. To determine how much magnesium will react, we must first determine
how many moles of O2 are present.
50.
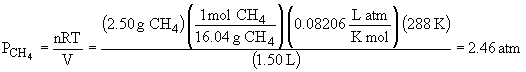
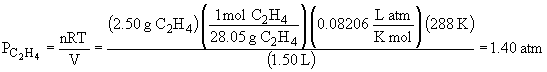
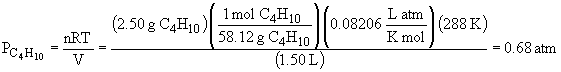
The total pressure is: Ptotal = PCH4 + PC2H4 + PC4H10 = 4.54 atm
54.
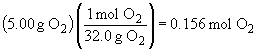
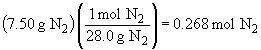
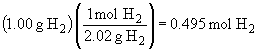
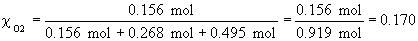
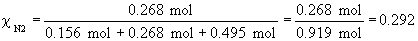
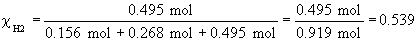
b)
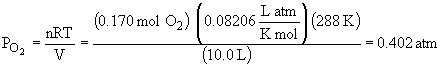
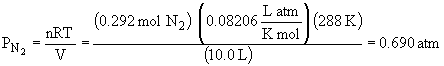
56.
99. This is a limiting reactant problem in the context of gaseous reactants.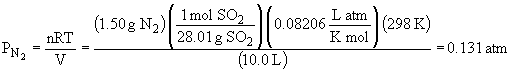
Ptotal = PN2 + PSO2 = 0.153 atm + 0.131 atm = 0.284 atm
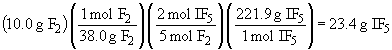
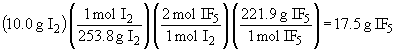
So, I2 is the limiting reactant and 17.5 g of IF5 will form.
The partial pressure of IF5 is:
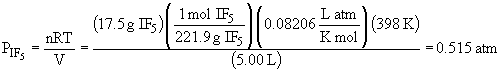
To find the mole fraction, the number of moles of F2 that remain needs to be determined:
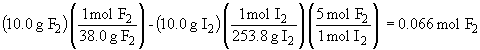
The mole fraction is then:
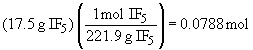
Chapter 11: Phase Changes
44a) 1.4 atm is equal to 1064 torr (pardon the significant figures violation). The temperature at which the vapor pressure of water is approximately equal to this pressure is about 110° C.
b) Ethyl alcohol will boil at 60° C when
the external pressure is approximately 350 torr.
46. Water will boil in Reno at approximately 97° C.
52c. O2 will not sublime at 1 atm; the liquid phase is stable
over a well defined temperature range.