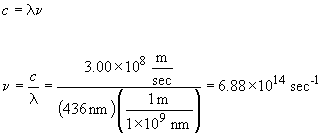
Review Assignment #6
Chapter 6: Electronic Structure of Atoms
4 a.False. Electromagnetic radiation is
capable of passing through water. (That's why you can see through it.)
b. True.
c. True.
d. False. The glow from a fireplace
and the energy within a microwave oven are forms of electromagnetic radiation;
the blast from a foghorn is not.
6. Beginning with the shortest wavelengths, the order
will be: X-Rays < orange light < infra-red (from the toaster oven)
< radio wave < 50 Hz radiation
9. 436 nm light is in the blue portion of the visible spectrum.
16. The relationship between energy and frequency
of a given photon is given by E = hn.
For the AM radio wave:
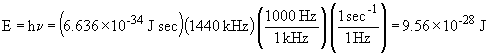
For the FM radio wave:
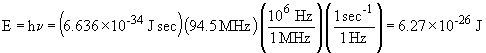
So, the FM radio waves are more energetic by about two orders of magnitude.
19. First, find the frequency that corresponds to
the energy required:
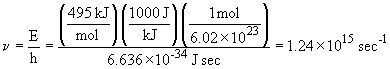
The corresponding wavelength is:
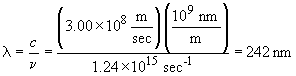
This light is in the ultraviolet portion of the electromagnetic spectrum.
27a. The energy of an emitted photon is given by:
33. The wavelength of a solid object in motion is given by: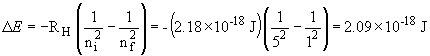
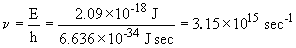
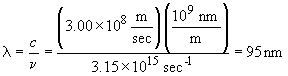
This photon is emitted by the n = 5 ®n=1 transition; it is deep in the UV.
b.
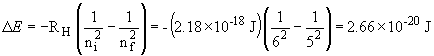
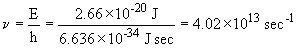
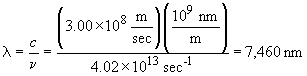
This photon is emitted by the n = 6 ®n= 5 transition; it is in the infra-red.
c. The energy of an absorbed photon is equal in magnitude but opposite in sign to that of an emitted photon. So,
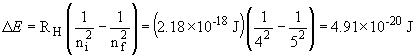
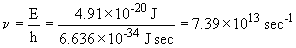
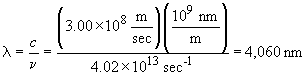
This photon is absorbed and results in the n = 4 ® n= 5 transition; it is in the infra-red.
40. The probability of finding an electron at any point in space is proportional to the square of the wavefunction amplitude, y2.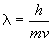
a.
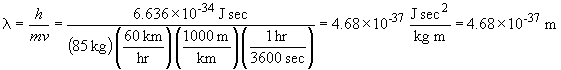
A note on units: the definition of a joule is:
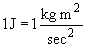
So in the above problem, a conversion factor can be used to cancel joules:
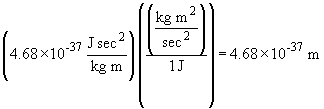
b.
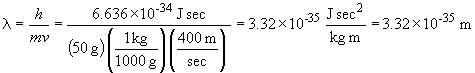
c.
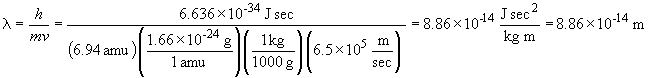
42 a. When n = 3, possible values of l and ml are:
l: 0, 1, 2
ml = -2, -1, 0, 1, 2
b. When n = 5, possible values of l and mlare:46.l: 0, 1, 2, 3, 4
ml = -4, -3, -2, -1, 0, 1, 2, 3, 4
|
|
|
|
|
comment
|
|
|
|
|
|
|
|
Not allowed; l cannot be greater than n - 1; these quantum numbers describe a non-existent "2d" orbital. |
|
|
|
|
|
|
These quantum numbers describe a 4f orbital (allowed) |
|
|
|
|
|
|
These quantum numbers describe the 1s orbital |
|
|
|
|
|
|
Not allowed; ml must be an integer in the range -l ³ml³l; the n and ml quantum numbers would describe a 6f orbital if ml had an allowed value. |
50. (a) The number of nodes for any orbital is always
equal to n - 1, where n is the principal quantum level. (b) So, there will
be one node in a 2px orbital and there will be two nodes
in the 3s orbital. (c) The angular nodes in p orbitals are defined
by two dimensional planes that include the nucleus; therefore there will
be a region of electron density above the plane, another below the plane,
but no electron density in the plane itself. (d) In the hydrogen atom,
the energies of the orbitals depends solely on the principal quantum number.
So the order of energies for the listed orbitals would be: 2s =
2p
< 3s < 4d < 5s.
60a) when n = 2, there are four possible orbitals: the 2s (where l = 0, ml = 0) and three 2p orbitals (l = 1, ml = -1, 0 and 1). There are four electrons that can have n = 2 and ms = -½; that is, we are only counting electrons with "down" spins.
b) When n = 5 and l = 3, there is a total of seven orbitals possible (ml = -3, -2, -1, 0, 1, 2, 3; these are the 5f orbitals); since each orbital can have two electrons, there is a total; of fourteen electrons with n = 5 and l = 3.c) The three quantum numbers n = 4, l = 3 and ml = -3 define a single 4f orbital. Only two electrons can have the same n, l and ml values.
d) The three quantum numbers n = 4, l = 1 and ml = 1 define a single 4p orbital. Only two electrons can have the same n, l and ml values.
64. Only a is correct. The correct orbital
diagrams are:
| a) | Sr | [Ar] | ¯ | | __ | __ | __ | __ | ||||||
| 4s | 3d |
| b) | V | [Ar] | ¯ | _ | _ | _ | ___ | __ | ||||||
| 4s | 3d |
| c) | Si | [Ne] | ¯ | _ | _ | __ | |||||
| 3s | 3p |
| d) | Sn | [Kr] | ¯ | ¯ | ¯ | ¯ | ¯ | ¯ | _ | _ | __ | |||||||||
| 5s | 4d | 5p |
66.
|
|
|
|
|
|
|
1s22s22p63s23p64s2 ,or, [Ar] 4s2 |
|
|
|
1s22s22p63s23p63d104s24p2
or, [Ar] 3d104s24p2
|
|
|
|
1s22s22p5
or, [He] 2s22p5
|
|
|
|
1s22s22p63s23p63d74s2
or, [Ar] 3d74s2
|
|
|
|
1s22s22p63s23p63d104s24p64d35s2
or, [Kr] 4d35s2
|
|
|
|
1s22s22p63s23p63d104s24p64d104f55s25p66s2
or, [Xe] 4f56s2 |
74a.
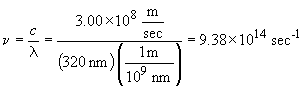
b. For one photon:
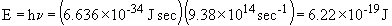
For one mole of photons,
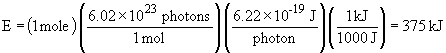
c. UV-B radiation is more energetic. It has shorter wavelengths and therefore greater frequency; energy is proportional to frequency.
d. Yes, since UV-B radiation is more energetic, it is more capable of causing chemical reactions that can damage living tissue.
79. For the reaction, O3 (g) + hn
® O2 (g) + O (g)
The change in enthalpy is given by:DH°reaction = S (DH°f)products - S (DH°f)reactants
DH°reaction = [DH°f(O2) + DH°f(O)] - [DH°f(O3)]
DH°reaction = [0 + 247.5] - [142.3] = 105.2 kJ/mol
This is the energy required to convert one mole of O3 to the corresponding products. The energy per O3 molecule is given by:
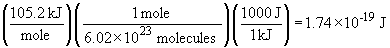
The frequency of light that corresponds to this energy is:
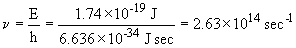
The wavelength is
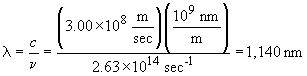
This wavelength lies in the infrared. This is much longer than expected since O3 absorbs UV radiation, not infrared. The mechanism involved is more complicated than a simple photon absorption and bond cleavage. See the discussion on page 689 for a more complete discussion of the role of ozone in filtering ultraviolet radiation in the atmosphere.
84a. The uncertainty in the velocity measurement is
given as 0.2%, or (0.002) (2.2 ´ 105
m/s) = 440 m/s. The resulting uncertainty in momentum, Dp,
is
the product of the electron mass and the uncertainity in the momentum:
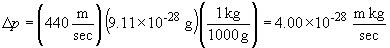
According to the Heisenberg Uncertainty Principle,
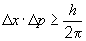
So, the minimum uncertainty in the position, Dx, can be found by rearranging the above relationship as:
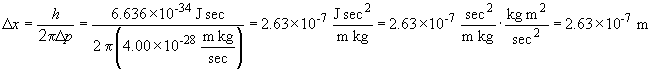
b. By the same process we have:
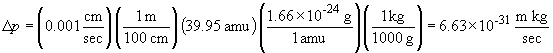
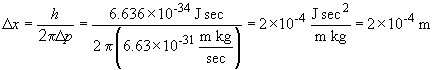
The relatively low uncertainty of the argon atom's momentum comes at the cost of a relatively large uncertainty in its position; note that 2 ´ 104 m is many orders of magnitude greater than the atomic radius of the atom itself.
Chapter 7: Periodic Properties of the Elements
13. (a) Atomic size decreases as you move from left to right across the periodic table due to increasing effective nuclear charge; that is, as the charge of the nucleus increases, it has a stronger attraction to the valence electrons. (b) Atomic size increases as you move from top to bottom in a given column as the valence electrons populate quantum levels of increasingly high energy. (c) The order of size, from smallest to largest is: F< S < P < As.
15. By the same reasoning as above, the helium atom will be smaller than the hydrogen atom since its nucleus has a greater charge and attracts the electrons more effectively. The helium atom is smaller than the neon atom because the neon atom has electrons in the second principal quantum level, which is more diffuse than the first.
18a. Ga ® Ga+ + e- DH = IE1
Ga+® Ga2+ + e- DH = IE2
20. To answer this question, you need to consider the location of the electron being removed in these cases:
b. Rh3+ ® Rh4+ + e- DH = IE4
Scandium:3rd Ionization energy: Sc2+ ® Sc3+ + e- DH = IE3 = 2,390 kJ/mol
4th Ionization energy: Sc3+ ® Sc4+ + e- DH = IE4 = 7,090 kJ/mol
The electron configuration of Sc2+ is: [Ar] 3d1. So the electron removed in the third ionization process comes from the relatively high energy 3d orbital. On the other hand, the electron configuration of Sc3+ is [Ar], or [Ne] 3s23p6. The electron removed in the fourth ionization process originates from a much lower energy 3p orbital, that it, the fourth ionization process is the first in which low-level core electrons are being removed. The very strong attraction to the nucleus of the core electrons accounts for the very much enthalpy change of the fourth ionization process compared to the third
Titanium
3rd Ionization energy: Ti2+ ® Ti3+ + e- DH = IE3 = 2,650 kJ/mol
4th Ionization energy: Ti3+ ® Ti4+ + e- DH = IE4 = 4,170 kJ/mol
In the case of titanium (named for the Titans, the giants who were born of the Greek earth goddess, Gaea, and who ruled the earth until overthrown by the Olympian gods), the electron configuration of Ti2+ is [Ar] 3d2. For Ti3+: [Ar] 3d1. In neither case does the removal of an electron involve the removal of a core electron; as a result, there is not the dramatic difference in IE3 and IE4 that was seen in the case of scandium. In case you're interested, the fifth ionization does involve the removal of a core and shows a large increase in ionization energy.
5th Ionization energy: Ti4+ ® Ti5+ + e- DH = IE5 = 9,570 kJ/mol
21. As you go down the Group 8A elements, the ionization
energies steadily decrease. This is because the valence electrons occupy
increasingly high energy quantum levels and are not as strongly attracted
to the nucleus. For the same reason, the atomic radii of the elements show
a gradual increase as you go down the column.
24 a) Ca will have a larger ionization energy than K since it has a larger effective nuclear charge.
b) C will have a larger ionization energy than Si since its valence electrons are in a lower energy (i.e., more stable) quantum level.c) Br will have a larger ionization energy than Mn due to its greater nuclear charge.
d) Xe will have a larger ionization energy than Sn due to its greater nuclear charge.
28. Recall that a negative electron affinity indicates
an exothermic (that is, favorable) process. To see why the addition of
an electron to bromine is favorable, consider the electron configuration.
62. The plot shows the sequential ionization energies of carbon. The processes involved are:
Br: [Ar] 4s23d104p5 Br- : [Ar] 4s23d104p6
In this case the added electron enters a 4p orbital, which is not filled in a neutral atom of bromine. A neutral atom of krypton, on the other hand, has a filled valence shell, so an added electron will enter the higher energy 5s orbital and will not be strongly attracted to the nucleus.
Kr: [Ar] 4s23d104p6 Kr- : [Ar] 4s23d104p65s1
1st Ionization energy: C ® C+ + e- DH = IE1
2nd Ionization energy: C+ ® C2+ + e- DH = IE2
3rd Ionization energy: C2+ ® C3+ + e- DH = IE3
4th Ionization energy: C3+ ® C4+ + e- DH = IE4
5th Ionization energy: C4+ ® C5+ + e- DH = IE56th Ionization energy: C5+® C6+ + e- DH = IE6The electron configuration of neutral carbon is: 1s22s22p2. Thus, the first four electrons removed are from the second principal quantum level. These show a gradual increase since the electrostatic attraction between the nucleus and the electrons increases as each sequential electron is removed. This is because every time an electron is removed, the electron-electron repulsion of the remaining electrons decreases and they are increasingly pulled toward the nucleus.The large increase in ionization energy is seen at the fifth ionization step because this process involves removal of a core electron from the first quantum level. These are much closer to the nucleus and are held very strongly.
67
(a) The first ionization energy of phosphorus is greater than that of sulfur. This is one of the "exceptions" to the general trend of increasing ionization energy from left to right across a given row. It is explained by the fact that phosphorus has no unpaired electrons in the 3p orbitals (its configuration is [Ne] 3s23p3; so each of the 3p orbitals is half-filled). Sulfur, on the other hand, has two electrons in one of the 3p orbitals (its configuration is [Ne] 3s23p4). As a result, sulfur has greater electron-electron repulsion in the doubly-occupied 3p orbital which more than offsets its greater nuclear charge.
(b) The electron affinity for nitrogen is lower for nitrogen than either carbon or oxygen. The electron configurations for these elements are:
C: [He] 2s22p2
N: [He] 2s22p3
O: [He] 2s22p4The additional electron added to carbon goes into an unoccupied 2p orbital and does not greatly increase electron-electron repulsion. In contrast however, an additional electron in nitrogen must enter a partially filled 2p orbital; since there is already an electron in the region of space, electron-electron repulsion is increased and this serves to decrease the electron affinity. In the case of oxygen, the added electron also must enter a partially filled orbital and also must increase the electron electron repulsion. However, the magnitude of this effect is expected to be the same as that seen for nitrogen, but this is more than offset by the increase in nuclear charge which serves to attract the electron to the atom.
(c) The electron configurations for O+ and F+ are shown below:
| O+ | [He] | ¯ | | | | ||||||
| 2s | 3p |
| F+ | [He] | ¯ | ¯ | | | ||||||
| 2s | 3p |
The removal of an electron from F+ involves taking an electron from a filled orbital, that is, one that has significant electron-electron repulsion. The removal of an electron from O+ does would involve the removal of an electron from a half-filled orbital; there is no electron-electron repulsion making acting to make this process more favorable. This is entirely analogous to the fact that the first ionization of oxygen is lower than the first ionization energy of nitrogen.(d) The electron configurations of Mn2+, Cr2+ and Fe2+ are shown below.
| Cr2+ | [Ar] | __ | | | | | __ | |||||||
| 4s | 3d |
| Mn2+ | [Ar] | __ | | | | | | |||||||
| 4s | 3d |
| Fe2+ | [Ar] | __ | ¯ | | | | | |||||||
| 4s | 3d |
Mn2+ has a higher effective nuclear charge than Cr2+, explaining its higher third ionization energy. Fe2+ has paired electrons in a 3d orbital; this gives rise to electron-electron repulsion which makes ionization more favorable.
72 (c) Rn should have the highest ionization energy.
(d) Fr will have the lowest first ionization energy. (e) At will have the
most negative electron affinity. (f) Fr will have the largest atomic radius.
78 a) magnesium nitride = Mg3N2
b) The reaction is: Mg3N2 + 3 H2O ® MgO + 2 NH3Chapter 8: Introduction to Chemical Bondingc) The total number of mass of magnesium can be found from the mass of pure MgO that is obtained after the reaction of the mixture with water:
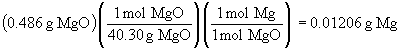
By the conservation of mass, this is equal to the number of moles of Mg in the mixture. We can define the mass of the two components in the mixture as follows:
Let x = mass MgO
Let y = mass Mg3N2
The total number of moles of magnesium can be related to the masses of the two components by:
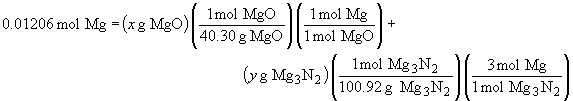
But since x + y = 0.470, we can substitute for y in the above expression, giving us one equation and one unknown, which we can solve.
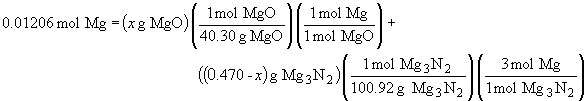
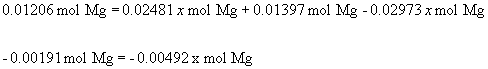
x = 0.388
Thus, the mass of MgO is 0.388 g and the mass of Mg3N2 is 0.082 g.
d) 3 Mg + 2 NH3 ® Mg3N2 + 3 H2
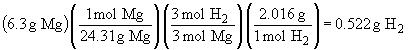
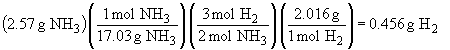
Since complete reaction of the ammonia yields the smaller amount of hydrogen in the above calculation, NH3 is the limiting reactant and 0.456 g of H2 will be produced.
e) DH° = [DH°f (Mg3N2) + 3 DH°f (H2)] - [3 DH°f (Mg) + 2 DH°f (NH3)]
DH° = [(-461.08 kJ) + 3 (0 kJ)] - [3 (0 kJ) + 2 (-46.19 kJ)] = -368.7 kJ
12. The lattice energies are: NaF (910 kJ/mol), MgO (3795 kJ/mol). This large difference is due to the stronger electrostatic attraction that exists between ions with charges of +2 and -2 (Mg2+ and O2-), compared with those of charges +1 and -1 (Na+ and Cl-). Recall that the potential energy of two ions is given by:
13. According to the equation above, the lattice energy of an ionic solid will (i) increase as the ionic charges increase and (ii) decrease as the sizes of the ions increase. The order of lattice energies based on the above considerations will be: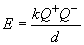
Here, Q+ is the charge of the cation, and Q- is the charge of the anion and k is a constant. So, provided the distance between the ions is similar, the potential energy of the MgO ion pair would be greater than that of NaCl by a factor of four. This is reflected in the lattice energies.
The smaller difference in lattice energies for SrCl2 and MgCl2 is accounted for by the fact that Sr2+ is a larger ion than Mg2+; thus the value of d in the above equation will be greater for SrCl2, resulting in a smaller lattice energy
KBr < KCl < LiCl < CaO19. This is a Hess's Law problem, where the formation of RbCl is viewed as the sum of several steps, one of which is the formation of a crystal lattice from gaseous ions.This is in agreement with the tabulated data.
The steps are:
Vaporization of Rb Rb (s) ® Rb (g) DH° = DH°f (Rb, g) = 85.8 kJ = DH1 Ionization of Rb Rb (g) ® Rb+ (g) + e- DH° = IERb = 403 kJ = DH2 Formation of Cl ½ Cl2 (g) ® Cl (g) DH° = DH°f (Cl, g) = 121.7 kJ = DH3 Electron Affinity of Cl Cl (g) + e-® Cl- (g) DH° = EACl = -349 kJ = DH4 Crystal Formation Rb+ (g) + Cl-(g) ® RbCl (s) DH° = DH5 = -DHlattice Formation of RbCl Rb (s) + ½ Cl2 (g) ® RbCl (s) DH° = DH°f (RbCl) = -430.5 kJ = DHtotal The last reaction is the sum of the first five, so the DH of the last reaction is the sum of the individual DH values for the component reactions. So,
DHtotal = DH1 + DH2 + DH3 + DH4 + DH5
Since DH5 is the unknown, we can rearrange the equation:
DH5 = DHtotal- DH1 - DH2 - DH3 - DH4
DH5 = -430.5 - 85.8 - 403 - 121.7 - (-349) = -692 kJ
This is the enthalpy change for the crystal formation reaction listed above. The lattice energy is defined as the change in enthalpy for the reverse reaction,
RbCl (s) ® Rb+ (g) + Cl- (g) DH = DHlattice = -DH5 = +692 kJ
This is less than that for NaCl, as would be expected given the larger size of the Rb+ ion compared to Na+.