 or,
or, 
Review Assignment #7
Chapter 8: Introduction to Chemical Bonding
2. (a) The octet rule states that atoms will tend
to react so as to have eight electrons in their valence shell. This "rule"
is often followed by ionic compounds, wherein atoms gain or lose electrons
to have a filled valence shell, and covalent compounds, in which atoms
share valence electrons to achieve a total of eight. (b) A neutral
sulfur atom must gain two electrons to fill its octet. (c) An atom
with the configuration 1s22s22p5
(e.g., a neutral fluorine atom) must gain one electron to fill its octet.
26. The stable monatomic ions of S, Cl and K all have
18 electrons (they are isoelectronic). (a) The size of the neutral
atoms follows the order: K > S > Cl. (b) The sizes of the ions is,
S2-> Cl- > K+. (c) The trend in ion sizes
can be understood by the fact the electron-electron repulsion for these
is equivalent, but the higher nuclear charge acts to pull the electrons
closer to the nucleus. In the neutral atoms, Cl is smaller than S due to
the higher effective nuclear charge. K is larger than either of these because
it has electrons in a higher quantum level and has a much lower effective
nuclear charge.
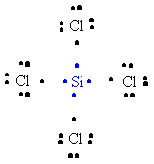
32. The formation of SiCl4 can be represented as follows:
 or,
or, 
34. CS2 has two double C-S bonds which tend to shorten the bond lengths relative to single bonds.
![]()
38. The order of electronegativities is:
a) O > S > PNote that these can be predicted by finding the elements that are closest to the upper right-hand corner of the Periodic Table.
b) Si > Al > Mg
c) C > Be > Mg
d) Br > Te > In
40. The greater the difference in electronegativity between the two atoms, the more polar will be the bond.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
So, the order of bond polarities will be:46.a) Be-F > C-F > O-F
b) O-Br ~ P-Br > N-Br
c) B-F > N-O > C-S
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* The "dividing" line between polar covalent and ionic compounds is usually defined as a difference in electronegativity of about 1.9. Compounds with a difference of 1.9 will have significant characteristics of both types of compounds.
48.
|
|
PCl3 | 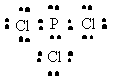
|
|
|
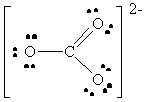
O22- | 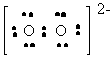
|
|
|
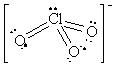
NO2+ | 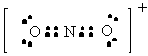
|
|
|
PO33- | 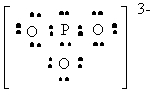
|
|
|
H2CO | 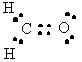
|
|
|
C2H2 |
52.
a) HNO3
b)
c) N2O4
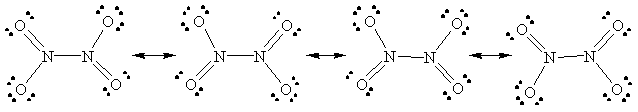
54.
Since the shorter bonds are associated with higher bond orders, the order of bond distance will be: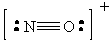
NO+: Bond Order = 3
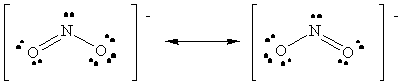
NO2-: Average Bond Order = 1 1/2
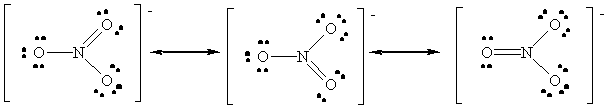
NO3-: Average Bond Order = 1 1/3
NO+ < NO2- < NO3-.
60.
|
|
NO2 | 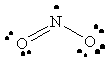 |
nitrogen violates the octet rule since it does not have a full octet of valence electrons |
|
|
ICl2- | 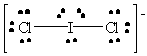 |
iodine violates the octet rule; it has ten valence electrons which is allowable since it has available d orbitals with which to make bonds. |
|
|
TeF4 | 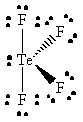 |
tellurium violates the octet rule since it has ten valence electrons |
|
|
BCl3 | 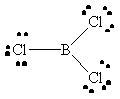 |
boron has an incomplete octet |
|
|
XeF4 | 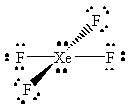 |
xenon has an expanded octet, with a total of twelve valence electrons |
62. Three important resonance forms for ClO2 are shown below. None of these satisfy the octet rule for all three atoms since there is an odd number total. In each of these structures, the octet of oxygen is satisfied, but chlorine has 11, 9 and 7 electrons in these structures.
77. The octet rule for all atoms (except H) in urea is satisfied in the structure below.
According to the electroneutrality principle, the most stable resonance form will be the one with the lowest formal charges. With this in mind, the resonance form on the far left will be the best representation of ClO2, that is, the molecule probably has two double bonds.
85. The Lewis structure for I3-
is shown below. Note that the central iodine atom has an expanded octet.
Fluorine, cince it is in the second row of the periodic table (and therefore
has only 2s and 2p valence orbitals) cannot promote electrons
into d orbitals and cannot expand its octet. So an analogous structure,
F3-, cannot be drawn since F must follow the octet
rule.
Chapter 9. Molecular Geometry
14.
|
|
Structure |
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
 |
|
|
|
|
|
|
 |
|
|
|
|
|
|
 |
|
|
|
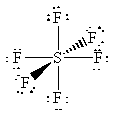
16. The following table summarizes the information on
AF4 molecules. Note that there are several elements that can
give rise to the same structure. For example, SF4, SeF4
and TeF4 will all have the "see-saw" geometry. To determine
the possible identity of the central atom, count the number of electrons
associated with the central atom, that is all of the non-bonding electrons
plus half of the bonding electrons. This will give the column number in
the Periodic Table. So, for example, the see-saw structure has 1 lone pair
and 4 bonds. This gives a total of six valence electrons from the central
atom, so any element in column 6 (except for oxygen - why?) is a possibility
for "A" in AF4.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
20.
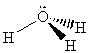
a) H-O-N angle: 109.5° (oxygen has a steric number of four)O-N-O angle: 120° (nitrogen has a steric number of three)
b) H-C-H angle: 109.5° (left carbon has a steric number of four)
C-C-O angle: 120° (right carbon has a steric number of three)
c) H-N-H angle: 109.5° (nitrogen has a steric number of four)
N-O-H angle: 109.5° (oxygen has a steric number of four)
d) H-C-C angle: 109.5° (left carbon has a steric number of four)
C-C-N angle: 180° (right carbon has a steric number of two)
63. The structures of the molecules are shown below:
While they all have the general formula, AF4,
the number of lone pairs on the central atom is different in each case.
Since the number of lone pairs will significantly affect the geometry,
these molecules all have very different shapes. SiF4 is tetrahedral,
SF4 has a "see-saw" shape, and XeF4 is square planar.
Chapter 11. Intermolecular Forces
8. (a) CHCl3 has the largest dispersion forces (it is the largest species); (b) only NH3 can hydrogen bond; (c) Ar has no dipole-dipole forces.
10. (a) To boil water, intermolecular hydrogen bonds must be overcome. (b) To melt KCl, the electrostatic attractions between the ions must be overcome. (c) To sublime I2, dispersion forces between the non-polar I2 molecules must be overcome. (d) To vaporize CH3Cl, dipole-dipole attractions must be overcome.
17. A molecule will form intermolecular hydrogen bonds with other molecules of the same kind if it has either N-H, O-H or F-H bonds. Only CH3NH2 and CH3OH have these bonds so only these species will undergo hydrogen bonding.
20. (a) HF has the higher boiling point since it can undergo hydrogen bonding, but HCl can not. (b) CHBr3 will have the higher boiling point since it is a much larger molecule and will have larger dispersion forces. (c) ICl will have the higher boiling point since it will have dipole-dipole attractions, whereas the non-polar Br2 will not.
21. The unusually high melting point, boiling point and
heat capacity of water are all due to the existence of hydrogen bonding.
Other manifestations of these forces include that fact that solid water
(ice) is less dense than liquid water (a very unusual property) and the
very high heat of vaporization of water.