What’s Cookin LAB 5 Fall 2005
Synthesis
of Salicylic Acid
Objectives
1) To acquaint you with some of the glassware
routinely used by an organic chemist.
2) To learn how to reflux a mixture and filter
a precipitate.
3) To synthesize salicylic acid from methyl
salicylate.
4) To
learn how to locate and use solubility data.
5) To learn how to do melting points.
6) To understand the concept of the LD50 scale.
7) To
become comfortable using the molecular drawing program ChemdrawÔ.
Introduction
Organic synthesis often
involves converting one functional group to another. When you perform the experiment today, you will convert the
functional group known as an ester into a functional group known as a carboxylic
acid. The product of the reaction,
salicylic acid should be a white precipitate, the starting material, methyl
salicylate is a liquid. Organic
synthesis involves changing part of a molecule by design. This results in a different molecule often
with different physical properties. By
changing part of a molecule you change the way it interacts with itself and
with other molecules. Intermolecular interactions govern both molecular and
macroscopic properties and behavior.
Molecular structure gives insight on how a molecule will interact with
other entities. Organic synthesis
allows us to manipulate molecular structure and hence, have some say on how a
molecule behaves.
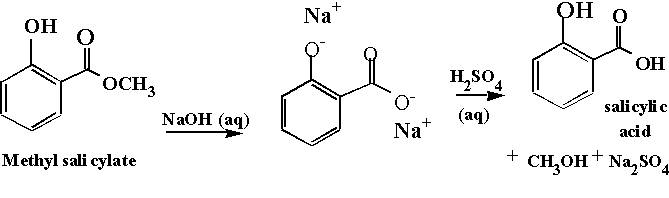
Figure 1 shows
our starting material. Note the
characteristics of the ester. Esters
are often liquids (oils) at room temperature.
At room temperature they are volatile to varying degrees and can be
quite pleasing to the nose since they smell nice. Esters isolated from plants are utilized for chewing gum
flavoring and other things. In our
particular case methyl salicylate should remind you of something found in the
wintergreen plant or birch tree. Oil of
wintergreen is 96-98% methyl salicylate.
In fresh wintergreen, methyl salicylate exists as the aglycone of the
glycoside gaultherin. Gaultherin also
contains primeverose, which is a disaccharide consisting of glucose and
xylose. Glycosides will be covered
later. This one would be a fun one to
go after.
The particular organic acid that we will make (synthesize)
is salicylic acid, shown in Figure 1. Carboxylic acids tend to be solids at room
temperatures due to the intermolecular forces (hydrogen bonding) present in the
carboxylic acid functional group. In
particular it is the OCH3 to OH conversion, which is
significant. Salicylic acid is the
"precursor" to aspirin.
Aspirin is a relatively simple aromatic compound that has served to ease
pain.
Figure
1.
The reaction we will perform today is known as a saponification. Saponification is defined as a base-promoted
hydrolysis of an ester.
Note that in our reaction the
intermediate will not be a
carboxylic acid, but a carboxylate ion.
Since the carboxylate is an anion (negative charge) it has to have a
positively charged friend, a cation.
The cation is the metal ion of the hydroxide base used. In our case this will be a sodium ion. The final step in the reaction is
protonation of the carboxylate to yield the carboxylic acid. This is done by the addition of an external
acid, sulfuric acid, as shown in Figure 1.
This organic chemist loves reaction mechanisms. Shown below in Figure 2 is a possible reaction mechanism for today's
reaction. The final protonation step is
omitted.
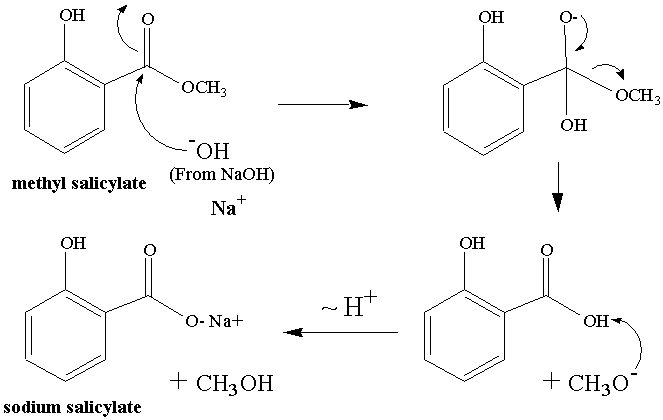
Figure
2: Mechanism of the reaction we will do today.
When doing an organic synthesis one must be able to judge
how well the reaction worked. One
method of doing this is to calculate the percent yield. For this reaction the % yield is defined as
(isolated salicylic acid/theoretical salicylic acid) X 100. To do this one must know how to deal with
moles. An effort many of you will have
to make here.
Pay attention to where things are stored throughout the laboratory. The lab in 3223 will be our lab space, it is a shared space. Get use to this room and where things are.
ChemdrawÔ is a program to assist
one in “drawing” chemical structures.
The Cal has version
5.0. Other programs exist, many of you
use them routinely, I am sure. I want
you to become comfortable using this or an equivalent program. You all will not get a tutorial on ChemdrawÔ. I want you
to utilize ChemdrawÔ at the level where you
can “cut and paste” molecules in and out of most “word” programs with little
effort.
PreLAb Reading: Review the techniques necessary
for this experiment. These techniques
include refluxing, filtration and melting points. Be sure you know the difference between a carboxylic acid and a
carboxylate ion. LD50 values determine toxicity, learn about these.
The Synthesis
Experimental:
Measure out about 4.0 mL of methyl
salicylate and record your weight to the nearest 0.01 gram. This can be done by pre-weighing a 100 mL
round bottom flask, adding the methyl salicylate, then weigh the flask
again. Add to this ~ 50 mL of 6.0 molar
NaOH. This has been prepared for
you. Place a magnetic stir bar in the
reaction flask and assemble this for reflux.
Make sure your condenser hoses are well connected. Allow myself to inspect your apparatus
before continuing.
With stirring adjust the heating
manual to about 1/2 power to get the reaction refluxing. Once refluxing, minimize power to maintain
reflux. The tuff part is getting the
mixture to stir. This may not occur
until the reaction mixture is heated.
Reflux for about 50 minutes and allow the reaction mixture to cool to
room temperature. Record all observations.
While the reaction mixture is cooling,
prepare an ice bath, which will replace your heating mantel. Remove the condenser from your cooled
reaction vesicle and carefully transfer the contents of the round bottom into a
250 mL Erlenmeyer. With stirring add about ~ 51 mL 3.0 molar H2SO4 (the same volume as base you added) slowly. Note all observations.
After this addition is completed, check the pH of your solution. Add more acid until the pH is ~ 1 or 2 (red to
litmus). Be sure to check that the
solution is acidic.
— U pNMN Strong
acid and base warning. NMNp
Allow
the mixture to sit for about 5 minutes then vacuum filter. Wash the crude solid with a small amount of
cold distilled water. Allow the solid
to be "sucked dry" for a couple minutes, depending on the line behind
you. Save a small amount of the crude
product and place in either a beaker or vial for drying and further
analysis. Recrystallize the rest of the
crude material from the minimal amount of hot (boiling) water and a small beaker. Use solubility data to get an estimate of how much water you may
need. Assume you had a quantitative
yield. After all is dissolved, decant
or gravity filter off any "junk" (usually not necessary) and allow
solution to cool to room temperature slowly.
After crystallization appears complete place flask in ice bath for five
to ten minutes, then suction (vacuum) filter.
Wash the crystals with a small amount of ice cold water.
Place product in the oven for
drying. Obtain melting points on both the crude and recrystallized products after they have dried. Obtain a mass of the final dry product. You must wait until things are dry to get
mass and map. Advice will be given
here, be attentive.
Lab Report: This should be about one to two pages and not include procedures or observations. These are in your notebook. Include in the write-up melting point data and comment on your % yield. A general statement on how the lab went and did you learn or like anything is welcomed. Include references for physical data (mp, solubility or LD50 values) & answer to following questions that can be answered directly in the space given except where noted.
Questions:
1) What was the mass and your percent yield of the recrystallized
salicylic acid?
2) From solubility data determine how much hot
water is needed to recrystallize 100.0 grams salicylic acid? What would be the maximum % of salicylic
acid you could expect to recover from this recrystallization. Show all work.
3) Using Chemdraw draw clear structures of the
molecules and reactions done in question 4.
4) Draw a balanced equation for the reaction of
salicylic acid with a. 1 eq. of NaOH b. 2 eq. of NaOH.