Flying insects
From Comparative Physiology of Vision
| Flying Insects | |
|---|---|
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Subphylum: | Hexapoda |
| Class: | Insecta |
Contents |
General Anatomy
In most insects there is one pair of large, prominent compound eyes composed of units called ommatidia (ommatidium, singular), of which there may be up to 30,000 ommatidia in a single compound eye. This type of eye gives less resolution than eyes found in vertebrates, but it gives acute perception of movement. There can also be an additional two or three ocelli, which help detect low light or small changes in light intensity. The image perceived is a combination of inputs from the numerous ommatidia, which are located on a convex surface, thus pointing in slightly different directions. Compared with simple eyes, compound eyes possess a very large view angle, and can detect fast movement and, in some cases, the polarization of light.[1]
Ocellus cross-section; Compound eye cross-section
Compound eyes
Compound eyes fall into two groups: apposition eyes, which form multiple inverted images, and superposition eyes, which form a single erect image. Compound eyes grow at their margins by the addition of new ommatidia.
A compound eye may consist of thousands of individual photoreceptor units or ommatidia. The image perceived is a combination of inputs from the numerous ommatidia (individual "eye units"), which are located on a convex surface, thus pointing in slightly different directions. Because the individual lenses are so small, the effects of diffraction impose a limit on the possible resolution that can be obtained (assuming that they do not function as phased arrays). This can only be countered by increasing lens size and number. To see with a resolution comparable to our simple eyes, humans would require compound eyes which would each reach the size of their heads.[2]
Dorsal ocelli
The term "ocelli" (singular ocellus) is derived from the Latin oculus (eye), and literally means "little eye". Two distinct ocellus types exist: dorsal ocelli (or simply "ocelli"), found in most insects, and lateral ocelli (or stemmata), which are found in the larvae of some insect orders.
Dorsal ocelli are light-sensitive organs found on the dorsal (top-most) surface or frontal surface of the head of many insects (e.g. Hymenoptera (bees, ants, wasps, sawflies), Diptera (flies), Odonata (dragonflies, damselflies) and Orthoptera (grasshoppers, locusts)). The ocelli co-exist with the compound eyes, thus most insects possess two anatomically separate and functionally different visual pathways.
The number, form, and function of the dorsal ocelli varies markedly throughout insect orders. They tend to be larger and more strongly expressed in flying insects (particularly bees, wasps, dragonflies and locusts), where they are typically found as a triplet. Two lateral ocelli are directed to the left and right of the head respectively, while a central (median) ocellus is directed frontally.
A dorsal ocellus consists of a lens element (cornea) and a layer of photoreceptors (rod cells). The ocellar lens may be strongly curved (e.g. bees, locusts, dragonflies) or flat (e.g. cockroaches). The photoreceptor layer may (e.g. locusts) or may not (e.g. blowflies, dragonflies) be separated from the lens by a clear zone (vitreous humour). The number of photoreceptors also varies widely, but may number in the hundreds or thousands for well-developed ocelli.
Two somewhat unusual features of the ocelli are particularly notable and generally well conserved between insect orders.
1. The refractive power of the lens is not typically sufficient to form an image on the photoreceptor layer.
2. Dorsal ocelli have massive convergence ratios from first-order (photoreceptor) to second-order neurons.
These two factors have led to the conclusion that the dorsal ocelli are incapable of perceiving form, and are thus solely suitable for light metering functions. Given the large aperture and low f-number of the lens, as well as high convergence ratios and synaptic gains, the ocelli are generally considered to be far more sensitive to light than the compound eyes. The ocelli are typically considered to be "faster" than the compound eyes. But compound eyes are better at detecting edges and are capable of forming images.
One theory of ocellar function in flying insects is that they are used to assist in maintaining flight stability. Given their underfocused nature, wide fields of view, and high light collecting ability, the ocelli are superbly adapted for measuring changes in the perceived brightness of the external world as an insect rolls or pitches around its body axis during flight. Other theories of ocellar function have ranged from roles as light adaptors or global excitatory organs, polarization sensors, and circadian entrainers.[3]
Ommatidium
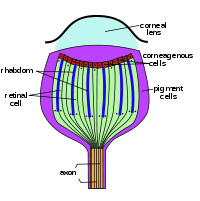
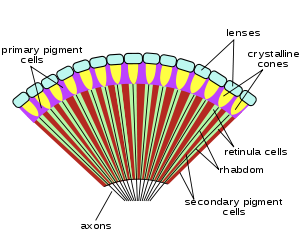
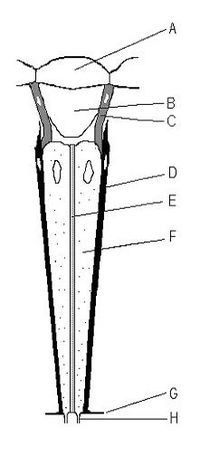
An ommatidium contains a cluster of photoreceptor cells surrounded by support cells and pigment cells. The outer part of the ommatidium is overlaid with a transparent cornea. Each ommatidium is innervated by one axon and thus provides the brain with one picture element. The brain forms an image from these independent picture elements. The number of ommatidia in the eye depends upon the type of insect and ranges from just a handful in the primitive Archaeognatha and Thysanura to around 30 thousand in larger Anisoptera dragonflies and in some Sphingidae among moths.
Ommatidia are typically hexagonal in cross section, and approximately ten times longer than wide. The diameter is largest at the surface, tapering toward the inner end. At the outer surface there is a cornea, below which is a pseudocone which acts to further focus the light. The cornea and pseudocone form the outer 10% of the length of the ommatidium. The inner 90% of the ommatidium contains long and thin photoreceptor cells that tightly pack the ommatidium. The portion of the photoreceptor cells at the central axis of the ommatidium collectively form a light guide, a transparent tube, called the rhabdom.
Since an image from the compound eye is created from the independent picture elements produced by ommatidia, it is important for the ommatidia to react only to that part of the scene directly in front of it. To prevent light entering at an angle from being detected by the ommatidium it entered, or by any of the neighboring ommatidia, six pigment cells are present. The pigment cells line the outside of each ommatidium. Each pigment cell is situated at the apex of the hexagons and thus lines the outside of three ommatidia. Light entering at an angle passes through the thin cross-section of the photoreceptor cell, with only a tiny chance of exciting it, and is absorbed by the pigment cell, before it can enter a neighboring ommatidium. In many species, in low-light situations, the pigment is withdrawn, so that light entering the eye might be detected by any of several ommatidia. This enhances light detection but lowers resolution.
The size of the ommatidia varies according to species, but ranges from 5 to 50 micrometres. The rhabdoms within them may cross-section at least as small as 1.x micrometres, the category of "small" being assigned in some cross-species studies to those under 2 micrometers.[4]
Unique Visual Optics
Bees
Microelectrode measurements from pairs of receptor cells in individual drone bee ommatidia show that the cells are electrically coupled, in contrast to cells in neighbouring ommatidia. By fitting the measurements to a model, it is found that coupling between drone retinulas is stronger than in the locust but weaker than in Limulus.[5]
The receptors in each fused-rhabdom ommatidium share identical receptive fields. The polarized-light (PL) response is always poor, but differences do sometimes occur between pairs of cells. Coupling can only partly account for the poor PL response in drone and locust cells. Receptors may uncouple at higher light intensities, since PL sensitivity increases slightly.[5]
Larger eye parameter implies heightened light sensitivity it is expected that the eye parameter of nocturnal bees is larger than that of diurnal bees.[6]
Small bees have larger eye parameters than large bees.
Flies
In certain flies, the rhabdom has separated into seven independent rhabdomeres. This has required the rewiring of the eye such that each ommatidium now has seven axons leading from it. The advantage to this arrangement is that it increases the number of picture elements by a factor of seven, without increasing the number of ommatidia.
In flies the visual pigment chromophore is 3-hydroxy retinal and an ultraviolet sensitizing pigment, 3-hydroxy retinol.[7]
Butterflies
Butterfly compound eyes have angular sensitivities narrower than expected from conventional apposition eyes.[8]
A basic set of ultraviolet-, blue- and green-sensitive receptors, encountered among nymphalid butterflies, forms the basis for trichromatic vision. Screening pigments surrounding the light-receiving rhabdoms can modify the spectral sensitivity of the photoreceptors so that the sensitivity peak is in the violet, yellow, red, or even deep-red, specifically in swallowtails (Papilionidae) and whites (Pieridae), thus enhancing color discriminability.[9]
A butterfly ommatidium contains nine photoreceptors.
A specialty of most butterfly eyes is the presence of a tapetum located proximally to the rhabdoms. Incident light propagates along the rhabdom, and when it is not absorbed it is reflected by the tapetum. The light travels then in the reverse direction and, if not absorbed, eventually leaves the eye again, where it is visible as eye shine (also called eye glow).[9]
Dragonflies
In dragonflies it has been demonstrated that the receptive fields of both the photoreceptors and the second-order neurons can be quite restricted. Further research has demonstrated that these eyes not only resolve spatial details of the world, they also perceive motion. Second-order neurons in the dragonfly median ocellus respond more strongly to upwards moving bars and gratings than to downwards moving bars and gratings. However this effect is only present when ultraviolet light is used in the stimulus; when ultraviolet light is absent, no directional response is observed. Dragonfly ocelli are especially highly developed and specialised visual organs, which may support the exceptional acrobatic abilities of these animals.[10]
Dragonfly median ocelli are shown to possess a number of remarkable properties: astigmatism arising from the elliptical shape of the lens is cancelled by the bilobed shape of the inner lens surface, interference microscopy reveals complex gradients of refractive index within the lens, the morphology of the retina results in zones of high acuity, and the eye has an exceedingly high sensitivity for a diurnal terrestrial invertebrate. It is concluded that dragonfly ocelli employ a number of simple, yet elegant, anatomical and optical strategies to ensure high sensitivity, fast transduction speed, wide fields of views and a modicum of spatial resolving power.[11]
The inner lens surface is bounded by darkly pigmented corneagenous cells which act as a mobile iris during light adaptation (Stavenga, Bernard, Chappell, & Wilson, 1979). Retinula cells lie directly behind the corneagenous cells; there is essentially no clear zone in dragonfly ocelli.[11]
Near the base of the rhabdom, retinula cells are cupped by reflecting tapetal cells, which appear brown in thick sections boundary.
Photo Transduction
General Phototransduction
When light hits rhodopsin the retinal cofactor is removed leaving the apoprotein metarhodopsin. The Gq-coupled protein receptor is activated following the conformational change of rhodopsin. The Gq receptor has three subunits; alpha, beta and gamma. The primary messenger subunit, alpha, is phosphorylated acquiring a nucleotide triphosphate group (GTP). As the protein signaler travels down the membrane of the cell it activates the membrane-bound enzyme Phospholipase C, known as, PLC. Activation of PLC enables this enzyme to perform an important hydrolysis reaction on the substrate phosphatidylinositol 4,5-biphosphate or PiP2 for short. Through the hydrolysis of PiP2, two products are formed inositol 1,4,5-triphosphate and diacylglycerol, IP3 and DAG, respectively. The formation of these products have created two important secondary messengers in this transduction process.
Excitation of the cell from this point forward in transduction is still debated. In Drosophila, Ca2+ and Na+ ion channels are activated when DAG is formed and/or when PiP2 is reduced by PLC. Also, light-gated transient receptor potential channels are activated. The opening of Ca2+/Na+ channels allows influx of these ions to occur causing depolarization of the cell and signaling to second order neurons that transport the signal to the brain.
In other species, cell depolarization is linked to submicrovillar cisternae (SMC). SMC are smooth ER Ca2+ stores. When IP3 is cleaved from PiP2, IP3 binds to channels on the SMC surface allowing an efflux of Ca2+ ions to be released into the cell. The spiked concentration of Ca2+ opens more and more Ca2+/Na+ ion channels as depolarization propagates along the plasma membrane of the cell.
Color Vision
It has been pointed out that the evolution of color in flowers has not been for our benefit, but for that of insects [12]. As such, investigation of color perception in insects promises to be rewarding. In 1882, an English naturalist named Sir John Lubbock conducted a series of experiments with light filters upon ants. His conclusions resulted in the first description of UV light perception in insects
[13] He wrote “It would seem, then, as a result of these experiments, that the limits of vision of ants at the red end of the spectrum are approximately the same as ours, that they are not sensitive to the ultra-red rays; but, on the other hand, that they are very sensitive to the ultra-violet rays, which our eyes cannot perceive”.
Today, while we have unearthed many of the mechanistic dissimilarities in color perception between insects and our own, Lubbock’s statement still stands. Humans cannot see UV spectrum, yet the perception UV spectrum appears in both sexual selection and plant advertisement in flying insects. [14]
Many studies of color vision in flying insects have been conducted as choice behavioral tests. Through this research, emergent similarities in trichromatic color vision have been found between nymphalid butterflies, moths and bees. Interestingly, these are all pollinators. These insects have nine classes spectra receptors (R1-9) within three ommatidial types. Six receptors with long-wave green sensitive pigments are consistent within these three ommatidial. However, there is heterogeneity due to differences in short-wave receptors. Within one ommatidial type, there is a UV receptor and a blue receptor. Another ommatidial type has two blue receptors and the third ommatidial has two UV receptors. [15][16][17] The property of the ninth spectra receptor found in the ommatidial is unknown. [9]
Behavioral studies in which insects choose between monochromatic light have been important in researching this topic. These investigations often involve positive reward training in foraging behavior. Two well studied representative species are the Japanese yellow swallowtail butterfly, Papilio xuthus, and the Honeybee, Apis mellifera. Trichromatic profile of Papilio xuthus show three maxima at approximately 430, 480, and 560 nm, while that of Apis mellifera is 345, 440 and 550 nm. [18][19] The radiation of photoreceptor spectral sensitivity may lead to heightened wavelength discriminatory power. Within Papilio xuthus, have been shown to discriminate at but 1 nm in wavelength change. This is the smallest discriminatory ability found in any species. However, the discriminatory power of Apis mellifera was found significantly less at 4 nm (courser then humans).
Neuronal Processing
While insects are not typically revered for the complexity, or size, of their brains, the immensely complex feats that they accomplish with such limited circuitry are a fascinating topic for research into neurology. For example, the brain of the honeybee has 0.01% of the number of neurons in a human brain, and it is still capable of facial recognition of humans! There is extensive debate over the necessary minimum level of organization and complexity required for interspecies facial recognition, and the simplicity of the honeybee’s visual processing pathways make them a prime candidate for research into this and other vision related behaviors and abilities [20].
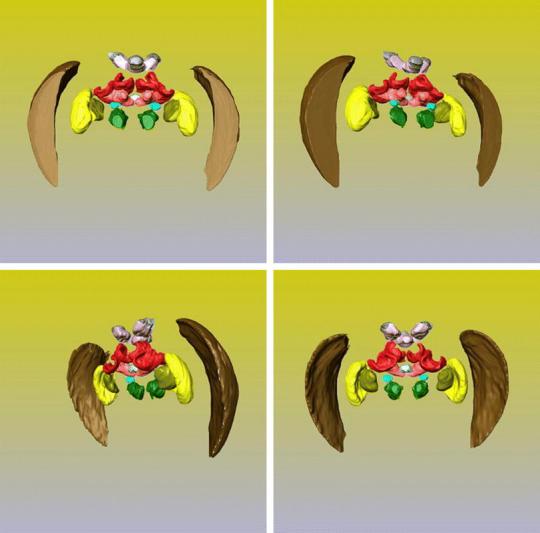
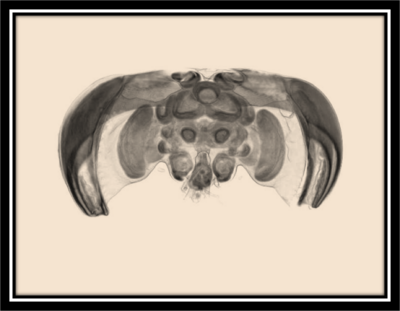
The higher order processing of visual cues begins with signal transduction from the compound eyes and ocelli of an insect to an ordered system of groups of neurons. Just as in primate sensory perception and processing, these areas have very specific visual activity and processing functions and are separated into a lobe-like structure and denoted ‘neuropiles’. The neuropils involved with vision are referred to as optic lobes specifically and are clearly pictured here (from a bee) both in an x-ray (of a bee brain in the head space) and in a cartoon (the yellow, brown, and olive parts are the optic lobes) to show more clearly the separation of functionality. Organization of each optic lobe is broken up into three separate ganglia; these are the lamina (brown), the medulla (yellow), and the lobula (olive). Retinal axons from the compound eyes synapse in the lamina with large monopolar cells (LMCs) where they are grouped for integration in the medulla and lobula, this is typically a one to one pathway, presenting a preserved map of stimuli to the optic lobes[21], though there is a considerable amount of lateral inhibition as is explained next. The LMCs are hyperpolarized by chlorine ions that travel through histamine, transmitter from photoreceptors, triggered gates[22]. Generally though, local information is sent from the lamina to the medulla, and receptors responding to global stimuli, being the greater background and wide field vision, synapse through the lamina into the lobula. It is here that filtering and processing begins, in the lamina, medulla and lobula.
Filtering and Integration
Much of the filtering necessary for processing is due to the amount of time flying insects spend in motion. Not simple slow movements akin to grounded insects, but flight between speeds of 1 mph to 35 mph. This may not sound terribly fast, but to gain perspective, a car is approximately 15-20 feet long, while a flying insect is, at the largest, 4 inches in length; that’s 1/45 – 1/60 the size! Imagine a car that could travel at top speeds of 2000 mph, being in that car is what the visual stimuli that fast flying insects such as dragonflies, bees, and hawk moths experience and process regularly. As a result of motion, perceptions of self-motion, movement of environment or predator or prey, being so crucial to survival for these creatures, specialized systems are in place to maintain acceptable perceptive awareness. Most importantly in flight is the ability to process what is known as optic flow, or the steady shift of a stationary environment across the retina as an observer travels through it[23]. Flying insects have a special mechanism in the medulla to filter out the spatial shifting and temporal aspects of optic flow to pass on reliable, streamlined information to the calyces about landmarks, food sources, prey, orientation and speed. This is done in the first two layers of the medulla by extensive interneurons, which work to inhibit large numbers of distracting signals and accessory information. The calyces, of which there are two each of two types, median and lateral, are part of a structure called the mushroom body, which is an integration center for all of the sensory information, to include connections to the motor specific neurons of the thoracic ganglia to control body movements, and connections which lead to memory and learning[21]. This integration is the most crucial point in higher order visual processing, most likely to be responsible for the keen ability of a bee to integrate optic flow speed into a distance travelled while simultaneously following known routes, recognizing landmarks, and remembering the proper vectors to the next landmark in the journey from previous flights[24]. It is also responsible for the ability to navigate tiny cracks and holes without error and through descending neurons to the thoracic ganglia provides optomotor functions, such as the success and smoothness of landings and avoiding collisions (though there is a special set of neurons, called the lobula giant movement detectors, which specifically respond to quickly growing images).
The Neural Pathways of Motion Detection
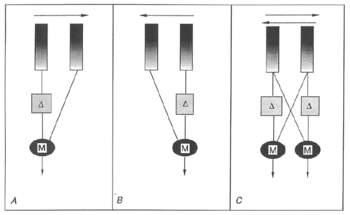
The current understanding of the mechanism of motion detection within the neural pathways of insects is based on a theory put forth by Hassenstein and Reichardt in 1956, called elementary motion detection (EMD). Because of the extremely small scale of the work, finding the neurons involved in this theoretical system has been a constant challenge to researchers who are still unable to describe the circuit via known neurons. The model is strongly supported however by behavioral, electrophysiological, and theoretical investigations and remains of import[25]. The EMD system begins in the Large (L) neurons of the lamina which are the second order neurons of the visual system. The L neurons collect graded potentials from individual ommatidia and then run to the medulla, which maintains a retinotopic architecture, where columns of neurons are representative of a single ommatidium. It is theorized that the EMD lies either within the medulla, or between the lamina and medulla.
The circuit of the EMD which allows for motion detection is fairly simple. It is based on a comparison between two appositional ommatidia, whose signals are correlated and then summed in a directionally selective cell[26]. In the first part of the system a single ommatidium relays a depolarizing signal towards the medulla. This signal is then split and sent to two symmetrical subunits (labeled M, for mirror, in the above illustration), one in the same column as itself, and the other in the column of the neighboring ommatidium. Before it reaches these subunits it is delayed in the pathway through its own column (denoted with a delta symbol in the above image). The signals from the subunits are then simultaneously passed on to a summing neuron which possesses directional sensitivity. As the signals from the ommatidium are sent through these processors one of the signals being delayed causes the summing neuron to send either a depolarizing or a hyperpolarizing signal (depending on its directional selectivity) to the lobula. The lobula combines these directionally selective signals from a wide field of view on to higher order neurons in the brain which control motor responses of the insect[27].
Ocellar Processing
The ocelli in flying insects are very pronounced compared with grounded insects and come in three eyes between the compound eyes (dorsally oriented, thus the distinction dorsal ocelli sometimes found) that serve primarily to provide simple light information and coordinate and orient flight through detection of differences in light, along with providing extra excitation to rapid changes in light with a quick response that cannot be directly linked to motor functions but serves to excite optomotor specific nerves in a summative type of signaling. This is found in three varieties[28]: a convergence onto a few second order neurons, followed by a large divergence to all systems, providing high sensitivity through the convergence. Another, found in bees, is a divergence to the other neuropils, adding information about light conditions to other stimuli, and in the case of a nocturnal bee helping to excite secondary neurons in the compound lamina to assist vision in dim light[29]. This direct wiring provides a faster response while sacrificing sensitivity. The third variety is a compromise, providing direct signals and converged signals. There are three ganglia (purple in cartoon), one for each ocellus, positioned on top of the brain, though the organization from there is variable as mentioned. A unique aspect of the neurons directly connected to the ocelli, are their diameter, some a stunning 25 μm, providing much faster signaling than all other neurons in some species[30].
Similarities with Vertebrates
Because neural structure and function is so similar in organisms, it comes as no surprise that the signaling methods are also similar. Signals above the retina are caused by various movements of neurotransmitters along with ions such as sodium, potassium, and calcium through voltage and chemically gated channels, to induce either depolarization or hyperpolarization in a hierarchy of neurons[24]. There is lateral inhibition in the lamina to create the same types of spatial and temporal antagonism that vertebrates experience via the center-surround organization. There is also convergence and divergence to organize information and integrate visual perception with other systems, just as vertebrates experience[31].
Higher Order Visual Perception
The archaic viewpoint of arthropods as simple reflex machines, experiencing their environment through a series of relatively simple sensorimotor responses has become grossly outdated as new experimental evidence points towards the rich complexity of higher order behavioral processing in invertebrates. Many flying insects, through extensive and complex perceptual capabilities, are able to detect and process their orientation in space, surrounding shapes, motion and subsequent navigation, and color, ultimately committing spatial maps and behavioral patterns to memory.[32]
Orientation and Shape recognition
Many flying insects recognize shape using both chromatic and achromatic cues. Achromatic stimuli are especially prevalent in the detection of objects through motion. Several insect species, ranging from flies, to hawkmoths have been shown to utilize relative motion to detect objects and infer object distance. Using relative motion, these species are able to discern edges through contrast detected by green-sensitive achromatic photoreceptors.[33]
Along with relative motion, several species of arthropods employ active motion to acquire information about their spatial surroundings. Many of these invertebrates employ an active vision strategy to aid in relative object location and range discrimination as the range in which stereoscopic vision can be used for depth perception is quite limited in arthropods. They also possess a relatively poor spatial resolution which largely prohibits object detection by contour outline alone.[34] In a sense, visual cues derived from motion are many insects only source of spatial information. As an example of active motion, locusts and mantids perform periodic lateral body movements and use the resulting translational motion information to assess the distance to a target.[35] Several species of bee have also been shown to use specifically tailored and structured flight patterns to provide them with motion parallax,or motion that serves to displace the position of an object viewed along different points, creating useful depth cues.[34]
Color Perception
For the detection of colored stimuli, flying insects, bees in specific, utilize both chromatic and achromatic visual systems.Both achromatic and chromatic systems are used alternatively depending on the visual angle given by the target. At larger visual angles (>15º), bees utilize the chromatic system. At smaller visual angles (<5º), achromatic green contrast is utilized. This presents a rather lucrative tradeoff. The achromatic vision allows the insect to enlarge its detection range of a stimulus while giving up the color appearance (S).Additionally; the green-contrast and the chromatic contrast pathways have differing angular size tuning. The green-contrast path conveys signals only of objects of small angular size with the chromatic path conveying only large angular size signals.[32] In honeybees, this dual processing is exemplified in flower detection. Before honeybees are able to analyze the color of a flower, they must first recognize it by means of green-contrast. The bee compares the long-wave signals from the flower itself with the long-wave signals from the background, illustrating the achromatic contrast of the object. Along with this achromatic contrast, actual color contrast through color opponent processing is essential in proper identification and preference of flowers. [32]
Vision Learning and Memory
With respect to cognitive function, the basic design of the invertebrate displays striking similarities to the larger more complex vertebrate brain. The existence of specialized neuropils and the presence of mushroom bodies, the higher order integration centers of the invertebrate brain within which information from various domains is integrated and processed, illustrates the basic neural architecture for cognition in the invertebrate brain. Neural plasticity and modulation can be found in the invertebrate brain, allowing for specific forms of learning and memory formation. [34][36]
A potent example of insect memory is the ability of insects to find their way back to places of significance using map-like memory formation.[34] When a honeybee departs on a foraging trip, it proceeds to acquire a visual representation, or map, of the nesting environment by performing an elaborate movement procedure. These learning flights, termed “turn-back-and-look” procedures have a conserved pattern across several species of wasp and bee. Ground-resting wasps, upon leaving the nest, turn back to face it then pivot around the nest in a series of arcs. During the pivots, height is exponentially gained above the ground at the same rate as the distance to the goal increases. This enables the nest entrance to be seen at a 45º angle below the horizon in the back of the wasp’s visual field. Simultaneously, the wasp turns against the pivoting direction so that the nest entrance is held in the front of the visual field. This map formation allows the insect to guide itself back to the nest upon its return[34]. It has been suggested that this elaborate spatial acquisition procedure serves to systematically link a series of “snapshots” taken at different locations relative to the nest, generate motion parallax information that could filter out shadow contours, distinguish close from distant landmarks, gaining information about absolute distance, culminating in a type of “flight simulation” guide for the return of the insect.[34]
Wavelength-Specific Behavior
Reflex behavior, as provoked by visual cues, plays a large part in insect behavior. Wavelength-specific behavior occurs when explicit wavelengths of light stimulate photoreceptors in the retina, resulting in specific reflex behavioral patterns. Phototaxis, an explicit example of wavelength-specific behavior exhibited in many species of flying insects, namely honeybees, is a strategy of light detection in which a bee, in an attempt to avoid predation, will reflexively seek out a path of movement in the direction of brightest light, irrespective of spectral content. This reflex is completely color-blind, utilizing only achromatic long-range receptor cells in the retina. Phototaxis is used as a method of navigation in dim light situations as well. If the sun itself is obscured by clouds, a bee will utilize the polarization pattern of the sky light to reconstruct the position of the sun, utilizing the sun as a sort of compass.[37]
Motion Detection
Motion detection is one of the most highly utilized functions of the insect brain. With their small number of neurons and comparatively simple neural circuitry, the speed and accuracy with which flying insects process and translate a cacophony of spatiotemporal input into evidence of self-motion and the surrounding environment is astounding. At the most basic level motion detection is a local process which compares changes in light intensity at neighboring points in the visual field.[38] Activation of only two neighboring photoreceptors in specific temporal order is sufficient to evoke directionally selective behavioral and neuronal responses. Consequently, motion is computed by a sequence of processing steps: First, spatiotemporal filtering of retinal light signals by local retinotopically organized signal pathways, second, detection of local motion signals by interaction between neighboring signal pathways, third, spatial integration of local motion signals over large parts of the visual field, and four, interactions of global motion signals between the two eyes.[32]
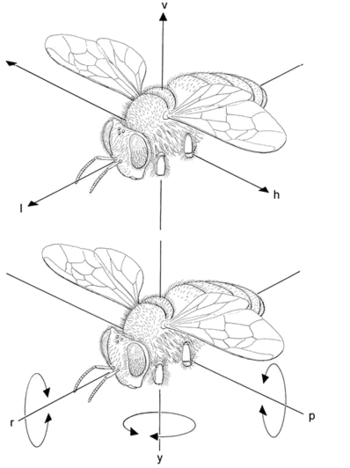
It is important to understand that motion detection in insects is largely based on patterns of optic flow across the eyes induced by self-motion during flight and other locomotion. There are two general descriptors for types of self-movement: rotation and translation[27]. Rotation can be further subdivided about three axes: rotation about the yaw axis (y axis), the roll axis (x axis), and the pitch axis (z axis). Translation can be divided into: vertical (up and down), longitudinal (forwards and backwards), and horizontal (side to side) movements. The patterns of optic flow across the eyes will be different for each degree of freedom. For example, rotation about the roll axis will cause the image on the eyes to move towards the top of the field of view (dorsally) for one eye and towards the bottom of the field of view (ventrally) for the other eye, while upward vertical translation will cause the image on both eyes to move ventrally. These movements are matched to directionally selective higher order neurons in the medulla, lobula, and lobula plate, which pass on the detection of motion directionality to influence motor responses in other parts of the body[26].
Classical behaviors guided by motion detection
Optomotor Response
During flight an insect may get off course from its intended direction. The optomotor response is a compensatory mechanism in which an insect will move in the same direction as motion across the eyes in an attempt to correct the misdirection[39]. For example if the insect is blown off course by a gust of wind causing it to veer right, the image on the eyes will move left causing the insect to turn left about the yaw axis[40].
Flight Stabilization During Pursuit
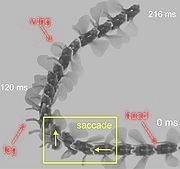
Insects can perform incredible feats of aerial acrobatics. In high speed maneuvers such as chasing prey or mates, or evading capture by predators, insects can move about all six degrees of freedom with extraordinary speed and agility sometimes at speeds up to several thousand degrees per second, and frequently at several hundred degrees per second[41]. Flight stabilization is theorized to be one of the functions of ocelli. Though the image plane lies well behind the retina and image resolution is very poor, the UV spectrum lies within the ocellar range of vision. As the insect moves about its axes the UV light from the sky is an easily detectable point of reference to reestablish level flight. The afferent axons associated with the ocelli are comparatively quite large, denoting the importance of speed of axonal transmission, a trait to be expected in a system so integral to self-movement[42]. During pursuit, forward and turning velocity are controlled by the angular motion of the target across the visual field. Quick saccades in which rapid turns about the body axis are made to pursue the target are determined by comparisons of retinal image flow between the two eyes which is processed by the motion detection units in the higher order neurons (See also Course Control below)[27].
Looming Cues to induce a landing response
A noted response of insects to wide-field motion detection or an expanding image (i.e. a large object, like the ground, moving towards the insect) is to stick its forelegs out in anticipation of landing. The visual system of flies have been shown to use relative rate of expansion of the image to determine time to contact in order to regulate deceleration of flight[40]. However, these looming cues are weak when the insect does not approach its landing site perpendicularly. Insects counteract this weakness by keeping their speed roughly proportional to the height above ground. This is accomplished by holding the retinal image velocity constant while approaching the surface. [43]
Peering to determine range of objects
Because insect eyes are immobile and unable to alter focus of image, they are consequently unable to infer distance by monitoring the refractive power needed for focus. Instead, they must use a mechanism called “peering” to determine how far an object is from themselves[40]. This behavior is often used to estimate the distance that an insect should jump to a nearby surface (in the case of crickets and locusts) or for determining distance to prey (praying mantids). More commonly known as motion or velocity parallax (which is common in humans, birds, and many other animals) an insect will (independently from the rest of the body) move its head in a translatory side to side movement. By doing this, the images of nearer objects will move faster across the field of vision and through a larger angle than an object that is more distant. The speed of retinal image motion as well as the speed of the head must be monitored by the brain in order to obtain distance information[44].
Navigation/Course Control/Centering Response
A major component of insect visual motion detection is the ability to regulate flight pattern and course control. It has been noted in studies of bees that speed of image motion across the eyes independent of direction is used to control navigation around obstacles and through small openings or tunnels[40]. A major component of insect visual motion detection is the ability to regulate flight pattern and course control. Both the speed of straight flight as well as the turning behavior characteristic of insect course control is mediated by the patterns of visual motion hitting both eyes[32]. Much like in motion parallax, the images of nearer objects will move across the retina with greater speed that distant objects. During centering it is hypothesized that the brain will balance the image speed on the two eyes by altering motor responses (i.e. flying closer to the image which is moving slower). This is also used to navigate around obstacles that they come into contact with during flight[45].
However, this optic flow not only helps to mediate a straight course of propulsion, but may also provoke turns in an instance to prevent collisions with obstacles. For example, as a fly approaches an obstacle, such as a textured wall, it generates sharp saccade-like turns to prevent it from crashing into the obstacle. The translational flow component necessary for spatial orientation depends on the relative distance to the textured wall, thus the saccadic flight strategy may help the insect nervous system to efficiently extract information about the overall spatial layout of the impending environment. [43]
Visual Odometry
The same mechanism of monitoring speed of image flow across the eyes is utilized in visual odometry to monitor distances travelled to and from food sources. Because directionality is three dimensional this method of information gathering can be somewhat unpredictable in its outcome, but is by and large successful for communicating directions to food sources to hive mates[27]. To alleviate this problem a bee instead of leaving a food source for the hive straight away will often turn around to face the place that it is leaving and perform what are assumed to be other types of distance and location measuring activities such as flying in consecutive increasing arcs. This saccadic flight may help to determine other spatial characteristics of the environment to record and report to hive mates[27].
Evolutionary Significance
Arthropods represent the only group of animals that have compound eyes. A large part of the evolutionary story of eyes stills remains a mystery and many discrepancies persist in the scientific community. One example of this is the common perception is that compound eyes, while losing greater resolution, gain superior motion detection. However, as Nilsson and Kelber point out in their paper A functional analysis of compound eye evolution, “an edge or object moves from pixel to pixel in the same way in camera eyes as it does in compound eyes.” However, others have proposed that the wide angle of ommatidia allows insects better motion detection but it remains unclear whether such an advantage evolved before or after the divergence between compound and non-compound eyes.[46] From the perspective of the pessimists the reason that the compound eye evolved, despite its disadvantages, may be that the structure was already well established before arthropods radiated or that the requirements for retinal epithelium for camera-like eyes is a difficult feat of evolution for creatures with exoskeletons. [47]
The presence of opsin predates the split between cnidarians and bilaterans (nearly 550 million years ago). Opsin has been found in archeal prokaryotes, unicellular eukaryotes as well as fungi. However, there is some evidence to suggest that prokaryotic opsin and animal opsin (or opsin-like molecules) are not homologous and represent an example of convergent evolution. The greatest step in this ancient gene’s evolution was the duplication and subsequent mutation of what can be called ur-opsin into two forms: c-opsin (ciliary-opsin;representing the folded ciliary membranes in mammal eyes for example) and r-opsin (rhabdomeric-opsin; representing the microvilli in compound eyes of insects and other arthropods for example). [48]
R-opsin is a G-coupled protein receptor that displays near exclusivity towards it coupling partners. R-opsin mostly couples with Gq subunits in its phototransduction cascade whereas c-opsin displays “promiscuity” among its coupling partners. Gq subunits are also found in vertebrate eyes in the forms of melanopsin that regulate circadian rhythms. Other types of opsins have evolved but are normally recruited as supportive elements and not as primary photoreceptors as c-opsin and r-opsin are. The major split among opsin and their preference of coupling partners suggests that the selective pressure(s) for r-opsin, expressed almost solely in the compound eyes of arthropods, must have been more rigid than the selective pressures felt by those carrying c-opsin. Another explanation for r-opsin’s near exclusivity may simply be a restraint by population genetics and genetic drift. However, due to a lack of phylogenetic information, it is hard tell the evolutionary story in full and thus to date no complete scenario has been devised. [49] [48]
While the presence of opsin predates arthropods themselves, according to fossil evidence, compound eyes appear along side the first arthropods in the early Cambrian period. Among the many species of insects, the basic plan of the ommatidium structure that makes up the compound eye is the same throughout the class with few exceptions suggesting a rather rigid selective pressure for its evolution compared to the compound eyes of other arthropod classes. For example, insects universally display one or two receptor cells with long visual fibers terminating in the 2nd optic ganglion and the remaining receptor cells with shorter fibers terminating in the 1st optic ganglion. Additionally. these receptor cells having different spectral sensitivities are thought to have been an ancient form of color vision. [47] It is common among non-insect arthropods to have inconsistent numbers of receptor types across classes and orders.
Most Insects use one or two chromophores in relation with opsin, either 11-cis-retinal (also utilized by primates) or the 3R and 3S enantiomers of 11-cis 3-hydroxyretinal. All but nine insect orders use 11-cis-retinal exclusively but those nine orders use either one or both. Both chromophores are derivatives of carotene which can be further broken down into Xanthophylls. Xanthophylls allow for the sysnthesis of 11-cis-hydroxyretinal. However, to derive Xanthophylls from carotene excess molecular oxygen is required. During the Carboniferous period, atmospheric oxygen was in higher concentrations than today. This increase of oxygen may have increased the reaction rate of xanthophylls and allowed insects to utilize both chromophores, a trait still maintained. [49]
Closest to insects in ommatidia structure are crustaceans, specifically malacostracan crustaceans. In both groups, a consistent ground plan of four cones cells and 8 receptor cells is present. Malacostracan Crustaceans and insects also share the presence of a crystalline cone. The advantage of a crystalline cone is that it allows long focal lengths in compact systems creating better resolution. Another impressive feature that is common to both groups is the wide range of optical function in the presence of a a fairly consistent cellular composition. In their paper A functional analysis of compound eye evolution Nilsson and Kelber note, “ The fact that evolution of new imaging mechanisms is realized within the common cellular plan, strongly suggests that optical sophistication evolved after the cellular composition was firmly established.”[47]
Due to the clear differences among focusing structures in insects/crustaceans and other arthropod groups, the common ancestor to all arthropods must have had very limited or no focusing structures at all being incapable of quick locomotion or any complex visually guided behavior. Thus, this ancestor would have been small or slow most likely predating the Cambrian explosion. Efficient compound eyes with focusing ability evolved independently in at least two branches of arthropods during the Cambrian explosion. [47] While details about this independent evolution are still missing, the evolution of focusing structures is a crucial event which lead to the ability of flying in insect.
Related Genetics among Insects and Other Metazoa
Along with opsin, flying insects also share conserved genes with other members of the animal kingdom in relation to eye development and as well as protein recruitment for crystallin lenses. A pattern of gene redeployment is seen for certain families of genes across genera. Notably the Pax gene family seems to have an underlying role in eye morphogenesis of many animals. Pax is older than eyes or even neurons and is thus said to have been “redeployed” in the case of visual development. Mutation of the Pax6 gene in Dorsphila has had ectopic consequences but the consequences of Pax6 mutation is not limited to insects. This widespread use of Pax family genes suggests that they may play an ancestral role in regulating differentiation genes. [50] [48]
Other Important genes that regulate insect eyes and their formation include Sine Oculis (Six) and members of the Orthodenticle-related homeobox gene family (Otx). Six is an evolutionary old gene family that, like Pax, predates the origin of eyes or nervous systems. While the details of its ancient role is fuzzy, evidence suggests that Six family genes play an important role in eye development. Mutations of such genes in Drosophila lead to loss of visual sense organs. Additionally, six1 and six 2 expresses at all levels of both the larval and adult visual systems for Drosophila. Otx genes have been shown to regulate opsin in Drosophila as well as genes involved in phototranduction cascades. Like the Pax and Six genes, Otx is conserved in both vertebrates as well as invertebrates as demonstrated by rescue experiments with Drosophila and mammals. It is believed that Otx genes pre-date the split between r and c-opsins. [48]
Final Questions
1.In detail, describe how motion parallax plays a part in the classical behaviors associated with movement detection in flying insects.
2.Compare and contrast the human eye with an apposition compound insect eye, including the advantages and disadvantages associated with the two types.
3. Describe the contrasting explanations for the evolution of the compound eye in arthropods.
References
- ↑ Insect morphology. http://en.wikipedia.org/wiki/Insect_morphology.
- ↑ Eye. http://en.wikipedia.org/wiki/Compound_eyes#Compound_eyes.
- ↑ Simple eye in invertebrates. http://en.wikipedia.org/wiki/Simple_eye_in_invertebrates.
- ↑ Ommatidium. http://en.wikipedia.org/wiki/Ommatidium.
- ↑ 5.0 5.1 Shaw, S.R. “Interreceptor coupling in ommatidia of drone honeybee and locust compound eyes.” Vision Research. 9, 9. (1969) 999-1029.
- ↑ Jander, Rudolf; Jander, Ursula. “Allometry and resolution of bee eyes (Apoidea)” Arthropod Structure & Development. 30, 3. (2002) 179-193.
- ↑ Hardie, Roger; Rudolph, Angela; Vogt, Klaus. “The compound eye of the tsetse fly (Glossina morsitans morsitans and Glossina palpalis palpalis).” Journal of Insect Physiology. 35, 5. (1989) 423-431.
- ↑ Nilsson, D.E.; van Hateren, J. H. “Butterfly Optics Exceed the Theoretical Limits of Conventional Apposition Eyes.” Biological Cybernetics. 57, 159-168 (1987).
- ↑ 9.0 9.1 9.2 Arikawa, Kentaro; Stavenga, Doekele G. “Evolution of color and vision of butterflies.” Arthropod Structure & Development. 35 (2006) 307e318.
- ↑ Simple eye in invertebrates.http://en.wikipedia.org/wiki/Simple_eye_in_invertebrates.
- ↑ 11.0 11.1 Berrya, Richard P.; Stangea, Gert; Warrant, Eric J. “Form vision in the insect dorsal ocelli: An anatomical and optical analysis of the dragonfly median ocellus.” Vision Research. 47, 10. (2007) 1394-1409.
- ↑ Backhaus, Werner. "Color Vision in Huneybees." Neuroscience and Biobehavioral Reviews 16 (1990): 1-12.
- ↑ Goldsmith, Timothy H. "Ultraviolet Receptors and Color Vision: Implications and a Dissonance of Paradigms.” Vision Res. 34.11 (1994): 1479-87.
- ↑ Osorio, D, and M Vorobyev. "A review of the evolution of animal colour vision and visual communication signals." Vision Research 48 (2008): 2042-51.
- ↑ Helversen, Otto Y. "Zur spektralen Unterschiedsempfindlichkeit der Honigbiene." J. comp. Physiol. 80 (1972): 439-72.
- ↑ Huglund, G, K Hamdorf, and G Rosener. "Trichromatic Visual System in an Insect and Its Sensitivity Control by Blue Light." J. comp. Physiol. 86 (1973): 265-79.
- ↑ Koshitaka, Hisaharu, Michiyo Kinoshita, Misha Vorobyev, and Kentaro Arikawa. "Tetrachromacy in a butterfly that has eight varieties of spectral receptors." Proc. R. Soc. 275.1637 (2008): 947-54.
- ↑ Helversen, Otto Y. "Zur spektralen Unterschiedsempfindlichkeit der Honigbiene." J. comp. Physiol. 80 (1972): 439-72.
- ↑ Koshitaka, Hisaharu, Michiyo Kinoshita, Misha Vorobyev, and Kentaro Arikawa. "Tetrachromacy in a butterfly that has eight varieties of spectral receptors." Proc. R. Soc. 275.1637 (2008): 947-54.
- ↑ Dyer, A. G., Christa Neumyer, and Lars Chittka. "Honeybee (Apis Mellifera) Vision Can Discriminate between and Recognise Images of Human Faces." Journal of Experimental Biology 208.24 (2005): 4709-714. Print.
- ↑ 21.0 21.1 Strausfeld, N. (1976) Atlas of an insect brain. Springer, Berlin Heidelberg New York.
- ↑ Hardie RC, Raghu P(2001) Visual transduction in Drosophila. Nature. 413:186-93.
- ↑ Borst A, Haag J (2007) Optic flow processing in the cockpit of the fly. In: Invertebrate neurobiology, North G, Greenspan RJ eds., CSHL-Press.
- ↑ 24.0 24.1 Borst A, Haag J (2002) Neural networks in the cockpit of the fly. J.Comp.Physiol.A 188, 419-437.
- ↑ Douglass, John K., et al. “Visual Motion Detection Circuits in Flies: Peripheral Motion Computation by Identified Small-Field retinotopic Neurons.” The Journal of Neuroscience. 1995: 5596-5611. Print.
- ↑ 26.0 26.1 Shoemaker, Patrick, David C. O’Carroll. “Insect-based visual motion detection with contrast adaptation.” Proceedings of Society of Photo-Optical Instrumentation Engineers. 2005: 292-302. Print.
- ↑ 27.0 27.1 27.2 27.3 27.4 Egelhaaf, Martin. “Insect Motion Vision.” 1 March 2008. Scholarpedia. Web. 1 Dec. 2011.
- ↑ Makoto Mizunami, Functional diversity of neural organization in insect ocellar systems, Vision Research, Volume 35, Issue 4, February 1995, Pages 443-452.
- ↑ Berry, Richard P. Ocellar Adaptations.The Journal of Experimental Biology 214, 1283-1293 © 2011. Published by The Company of Biologists Ltd.
- ↑ ^ Martin Wilson (1978). "The functional organisation of locust ocelli". Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology 124 (4): 297–316.
- ↑ Egelhaaf M, Kern R, Kurtz R, Krapp H.G, Kretzberg J, Warzecha A-K (2002) Neural encoding of behaviourally relevant motion information in the fly. Trends Neurosci. 25, 96-102.
- ↑ 32.0 32.1 32.2 32.3 32.4 http://www.neurobiologie.fu-berlin.de/menzel/Pub_AGmenzel/GiurfaMenzel_CurrOpinBiol_1997.pdf
- ↑ http://www.jneurosci.org/content/30/11/3896.full.pdf
- ↑ 34.0 34.1 34.2 34.3 34.4 34.5 North, Geoffrey, and Ralph J. Greenspan. Invertebrate Neurobiology. Cold Spring Harbor Laboratory Pr, 2007. Print.
- ↑ http://www.springerlink.com/content/pv483847t5866518/fulltext.pdf
- ↑ http://www.springerlink.com/content/c5hljtr2yatv7meg/fulltext.pdf
- ↑ Prete, Frederick R. Complex Worlds From Simpler Nervous Systems. The MIT Press, 2004. eBook.
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/2475948
- ↑ Gotz, Karl. “Flight Control in Drosophila by Visual Perception of Motion.” Kybernetik, June 1968: 199-208. Print.
- ↑ 40.0 40.1 40.2 40.3 Srinivasan, Mandyam, Michael Poteser , Karl Kral. “Motion detection in insect orientation and navigation.” Vision Research. 1999: 2749-2766. Print.
- ↑ Land, Michael F. “Visual Acuity in Insects.” Annual Review of Entomology. 1997: 147-177. Print.
- ↑ Wilson, Martin. “The Functional Organization of Locust Ocelli.” Journal of Comparative Physiology. 1978: 297-316. Print.
- ↑ 43.0 43.1 http://jeb.biologists.org/content/205/3/327.full.pdf
- ↑ Kral, Karl. “Side-to-side head movements to obtain motions depth cues: A short review of research on the praying mantis.” Behavioural Process.1998: 71-77. Print.
- ↑ Srinivasan, M. V. et. al. “Robot navigation inspired by principles of insect vision.” Robotics and Autonomous Systems. 1999: 203-216. Print.
- ↑ N. Franceschini, A. Riehle, A. Le Nestour, Directionally selective motion detection by insect neurons, in: D.G. Stavenga, R. Hardie (Eds.), Facets of Vision, Springer Verlag, Berlin, 1989, pp. 360–390.
- ↑ 47.0 47.1 47.2 47.3 Nilsson, Dan, and Almut Kelber. "A functional analysis of compound eye evolution." Arthropod Structure & Development. 36 (2007): 373-385. Print.
- ↑ 48.0 48.1 48.2 48.3 Vopalensky, Pavel, and Zbynek Kozmik. "Eye evolution: common use and independent recruitment of genetic components." Philosophical Transactions of The Royal Society. (2009): 2819-2832. Print.
- ↑ 49.0 49.1 Briscoe, Adriana, and Lars Chittka. "The Evolution of Color Vision In Insects." Annual Review Of Entomology . 46 (2001): 471-510. Print.
- ↑ Gehring, W.J. "New Perspectives on Eye Development and the Evolution of Eye and Photoreceptors." Journal of Heredity. 96.3 (2005): 171-184. Print.