Supernovae
and the Superheavy Elements
Richard C. Kohler
During the 15 billion years
that have elapsed since the big bang, from which we trace the origin of
the universe, a complex array of processes has shaped our present environment.
The emergence of galaxies, stars, planetary bodies, and eventually life
itself arose from the primordial big bang. This evolution represents
the consequence of interactions between natures basic forces and the fundamental
particles. Underlying the great diversity of our present day universe
has been the formation of the chemical elements. Chemical element
synthesis or nucleosynthesis is now understood in terms of nuclear
reaction that occur in a variety of cosmological settings. Within
this realm, the chemical elements are created. While scientist have
a good understanding of the processes that occur in the creation from hydrogen
through uranium, it is the transuranium and more specifically the super
heavy elements that remain the most illusive and least understood.
The transuranium traditionally refers to those elements that have an atomic
number greater than that of uranium.
Research over the last
30 years indicates there are three main sources responsible for the synthesis
of the chemical elements. They include (1) cosmological nucleosynthesis
in the big bang, (2) nucleosynthesis in the interstellar medium induced
by galactic-cosmic rays and (3) nucleosynthesis during stellar evolution
(Cox 89). A brief overview will cover
each of these processes with greater detail being given to those processes
necessary for the creation of the transuranium and the superheavy elements
(SHE).
Nucleosynthesis during the
Big Bang
The earliest era to which
we can trace the origin of our universe is that of the big bang, which
is believed to have occurred 15 billion years ago. Under the initial
conditions of the big bang, all matter existed and energy existed in the
form of a hot, dense fireball that contained only the elementary particles.
When the universe had cooled to 1010 K, it consisted of a sea
of photons, electrons, and positrons, neutrinos and antineutrinos, in addition
to the stalwart protons, and neutrons. These particles existed with
one another according to the following equation (Zin
79):
1H + e- Û
1n + n
1n + e+ Û
1H + n
As the universe cooled to a bone chilling 109
K, a more complex nuclei was formed. The universe continued to expand,
the temperature decreased to a point where 2H nuclei could survive
for a finite length of time. The following network of equations applied
during that period of the universe (Zin 79):
1H + 1n Þ
2H + g
2H + 1n Þ
3H + g ; 3H + 1H
Þ 4He + g
2H + 1H Þ
3He + g ; 3He + 1n
Þ 4He + g
3He + 4He Þ
7Be; 7Be + e-Þ
7Li + n
Within 3 minutes, the universe had expanded and cooled
to a point where nuclear reactions could no longer to be sustained.
The remaining neutrons then decayed to protons as follows (Zin
79):
The unreacted protons and neutrons from the big bang formed
the large residual hydrogen abundance that is seen in the universe today.
The primary nuclear reaction product of the big bang was 4He.
The hydrogen and helium elements that today comprise 98% of nature’s elements.
Nucleosynthesis in the Interstellar
Medium
While the big bang and stellar evolution can account for most of the elements
in the periodic table, there are a small number of elements that are produced
in the interstellar medium. Cosmic rays are very energetic nuclei, primarily
from H and He that permeate our galaxy. While their origin is poorly understood,
their properties have been studied extensively in balloon and satellite
flights above the Earth’s atmosphere. Li, Be, and B nuclei are believed
to be formed when cosmic rays interact with the 4He, carbon,
nitrogen, and oxygen nuclei present in the interstellar medium (Cox
89). These reactions occur at energies much higher than those characteristics
of the big bang or stellar evolution but in an environment which has a
very low density. The temperature is low and the Li, Be and B products
do not burn up after their formation, as they would in the stellar interiors.
Nucleosynthesis during Stellar
evolution
We know move on from the
big bang to the inner processes of stars. When the core of a star
reaches unusually high temperatures and densities, the protons in the core
acquire sufficient kinetic energy to overcome their mutual electric charge
repulsion and initiate nuclear reactions (Emp 91).
The process, known as hydrogen burning, characterizes main sequence stars.
Such stars burn protons into He by means of the following series of nuclear
reactions (Zin 79):
The hydrogen burning reactions stabilize a condensing star
and provide a vital source of energy by producing He from H. In order
to synthesize the more complex elements that provide the diversity of our
solar system, the advanced stages of stellar evolution must be explained.
As a main sequence star becomes older, it begins to develop into two phases:
1) a core composed largely of the He produced during hydrogen burning,
2) and an outer envelope consisting largely of unburned hydrogen (Emp
91)
If the mass of a star is sufficiently
large, the force of gravity begins to contract the core once again, leading
to still higher temperatures and densities. This causes the envelope
of the star to expand greatly and gives rise to a new stage in its evolution,
call the red giant phase. Stars that do not contain sufficient mass
to sustain more advanced stages of nuclear burning simply exhaust their
hydrogen fuel and undergo no further evolution. They are destined
to become white dwarf stars. Our on sun will eventually meet this
mundane end in 4 to 5 billion years.
During the red giant stage
of a star, gravitational pressure continues to compress and heat the core.
When the temperature reaches about 108 K, which corresponds
to a density of 104 g/cm3, a new type of nuclear
reaction becomes possible (Bau 89). The
exothermic reaction called He burning, is represented by the equation (Zin
79):
4He + 4He Þ
8Be + g (lifetime = 10-16seconds)
8Be + 4He Þ
12C + g
Once He burning begins, the core of the
star becomes stabilized against further gravitational contraction by the
evolution of nuclear energy, producing a new equilibrium situation.
At the same time oxygen can be produced via the reaction (Zin
79):
If the mass is sufficiently great,
a much more dramatic sequence of processes ensues. As a massive star
passes through the red giant phase, new core condition eventually develop.
For the most part, the core contains 12C and 16O
surrounded by envelopes composed of H and He. The electrostatic repulsion
arising from the large nuclear charges of 12C and 16O
inhibit nuclear reactions at He burning temperatures, leading to further
gravitational contraction and heating (Atk 91).
A star’s subsequent fate under these conditions is one of the least understood
phases of stellar evolution. The stellar core may continue to evolve
via processes similar to the equilibrium situations that exist in main
sequence and red giant stars, although on a much shorter time scale.
Explosive conditions may develop under which nucleosynthesis occurs very
rapidly, leading to supernovae explosions.
If the core temperature and
density reach approximately 500 * 106 K and 5 * 105 g/cm3,
new avenues of nuclear burning become available. One such reaction
involves the fusion of the 12C and 16O remnants from
He burning to form still heavier nuclei. These reactions are complicated,
but can be summarized as follows (Zin 79):
12C + 12C Þ
20Ne + 4He
16O + 16O Þ
28Si + 4He
12C + 16O Þ
24Mg + 4He
Because these reactions can occur
relatively rapidly at high temperatures, the evolution of the star proceeds
much faster at this stage and a more varied nucleus composition develops.
As the life cycle of a heavy star continues, a core composed largely of
nuclei near 28Si evolves. At temperatures near 109
K and densities approaching 106 g/cm3, a new process
referred to silicon burning begins (Bau 89).
These reactions involve both the ejection of an alpha particle (4He)
by high-energy photons present in the hot core and the inverse process
in which 4He is captured by the surrounding nuclei. It can be
summarized as (Zin 79):
This chain of reaction stops near
mass number A=56. Iron is nature’s most stable nucleus. When
an Fe core develops in a star, the ability of energy-liberating nuclear
reactions to provide support for resisting gravitational contraction becomes
limited. At this point, a rather complex unstable star has developed,
containing most of the elements up to Fe in various layers. At each stage
of evolution, the synthesis processes becomes less efficient and more diverse.
This accounts for the steady decrease in the abundance of the elements.
Due to the special ability of 56Fe nuclei, a sink is created
that accumulates ironlike elements, producing the abundance peak at Fe.
It also accounts for the low abundance of the heavier elements.
The accumulation of iron-group
elements in the core of stars with masses greater than 10 times the mass
of the sun lead to catastrophic conditions. Without the stabilizing
influence of nuclear energy evolution, the gravitational force causes the
core to collapse. The implosion occurs on a time scale as short as
seconds, during which the density of nuclear matter may reach 10^8 g/cm^3
with a corresponding temperature well over 109 K in the center
of the core (Bau 89). This rapid heating
is followed by a massive shock wave that leads to the explosion of the
star, a process believed to be associated with supernovae such as SN1987A.
There are two important consequences
of gravitational collapse and the rapid heating that follow: First, the
temperature increases triggers an extensive network of nuclear reactions
throughout the outer envelopes of the star. This leads to a diversity
of nuclear species for the elements previously formed. Second, the condition
in the very center of the core, where the temperature and density are highest,
cause the iron nuclei to break up by means of photodisintegration reactions,
leading to the following processes (Zin 79):
56Fe + g Þ
134He + 41n
4He + g Þ
21H + 21n
1H + e- Þ
1n + n
As far as nucleosynthesis is concerned,
the important point is that large numbers of neutrons are produced in the
central core region. Because neutrons have no electric charge, they
can interact with previously processed nuclear matter without the constraint
of electromagnetic repulsion. This further enriches the variety of
nuclei and produces nature’s heaviest elements.
This stage of nucleosynthesis is commonly
referred to as the r process (r is for rapid) and proceeds according to
the following series of abridged reactions (Zin
79):
56Fe Þ
57Fe Þ 58Fe Þ
Þ 79Fe
These reactions produce highly neutron rich nuclei that are
well submerged below the sea of instability. As neutron addition
continues, nuclear beta decay (conversion of a neutron into a proton within
the nucleus) becomes increasingly probable. This produces the next
higher element (Zin 79):
79Fe Þ
79Co + e- + n ; 79Co
Þ 80Co Þ
81Co; 81Co Þ 81Ni
+ e- + n
It is the r process that forms thorium
and uranium (Z=90 and 92 and may account for the existence of any of the
“superheavy” elements in nature. Unfortunately, it
now becomes necessary to move our investigation from the cascades of supernovae
to the inner workings of man-made accelerators.
Transuranium Research
By using neutron and
positive-ion bombardment, scientists have been able to extend the periodic
table. Prior to 1940, the heaviest known element was uranium (Z=92), but
in 1940 neptunium (Z=93) was produced by neutron bombardment of 238U.
The process initially gives 239U, which decays to 239Np
by b -particle production (Mas
88):
239U + 1n Þ 239U
Þ 239Np + -1e
In the years since 1940, the elements
with atomic numbers 93 through 112, have been synthesized. Many of these
elements have very short half-lives, as illustrated in Table 1.
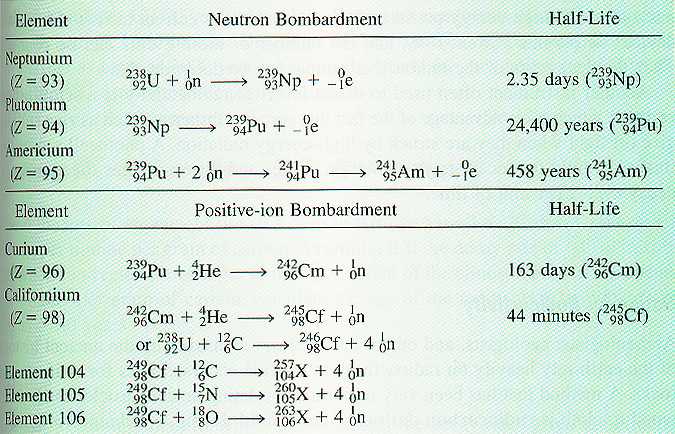 Table from Chemistry 3rd Edition,
A. Jackson
Table from Chemistry 3rd Edition,
A. Jackson
During the late 1940’s, it was predicted
that transuranium elements were expected to have a life span comparable
to that of uranium or thorium, and to be produced in macroscopic quantities.
For chemistry, it meant that the heavy elements would open up discoveries
for new compounds, and for physics it meant a better understanding of matter.
One question that arose as long ago as the early Sixties was whether such
shell effects in nuclei much heavier than uranium would cause them to be
stable enough that they might still occur in trace amounts in nature, or
could be synthesized. Early calculations from 1966 predicted an "island
of stability" in this region for the isotope 298114(Akt
91). This was the birth of the idea of the superheavy elements (SHE),
and marked the start of experimental efforts to synthesize them. But much
like life, their early optimism was dealt a setback when research indicated
that the superheavy elements were short lived and difficult to produce.
Many properties of transuranium elements
can be described by analogy with a drop of liquid. The classical liquid
drop model, which treats nuclear binding as an interplay of attractive
nuclear forces (acting between both protons and neutrons) keeping the nucleus
bound, and repulsive electrostatic forces ( acting only between protons)
driving apart. The drop model should give reliable predictions of nuclear
masses and binding energies (Sea 63). The binding
energy is the energy required to decompose a nucleus into it component
parts. However, it says nothing about the internal arrangement of protons
and neutrons in a nuclear drop(Sea 63). This
inner arrangement essentially determines the properties of a nuclear system,
for example, its exact binding energy. Figure 1 indicates the binding energy
per nucleon as a function of the mass number.
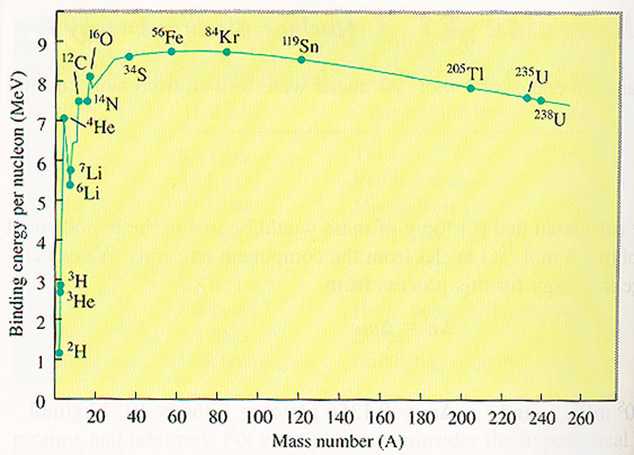 Graph from Chemistry 3rd Edition, A.
Jackson
Graph from Chemistry 3rd Edition, A.
Jackson
The most stable nuclei are at the top of the curve. The most
stable nucleus is 56Fe, which has a the highest binding energy
per nucleon of 8.79 MeV. Just like the electron cloud of the atom, atomic
nuclei also exhibit a shell structure. While the atoms of noble gases (He,
Ne, Ar, Kr, Xe, and Rn) owe their stability to closed shells of electrons,
certain atomic nuclei are extremely stable because they have closed shells
of neutrons and protons. This stability of nuclei with proton number Z
or neutron number N equal to 2, 8, 20, 28, 50 and 82 becomes evident in
two fundamental properties: the nuclear binding energies, as deduced from
nuclear masses, are exceptionally high for such so-called "magic number"
nuclei (Sea 90). For neutrons, N=126 is also
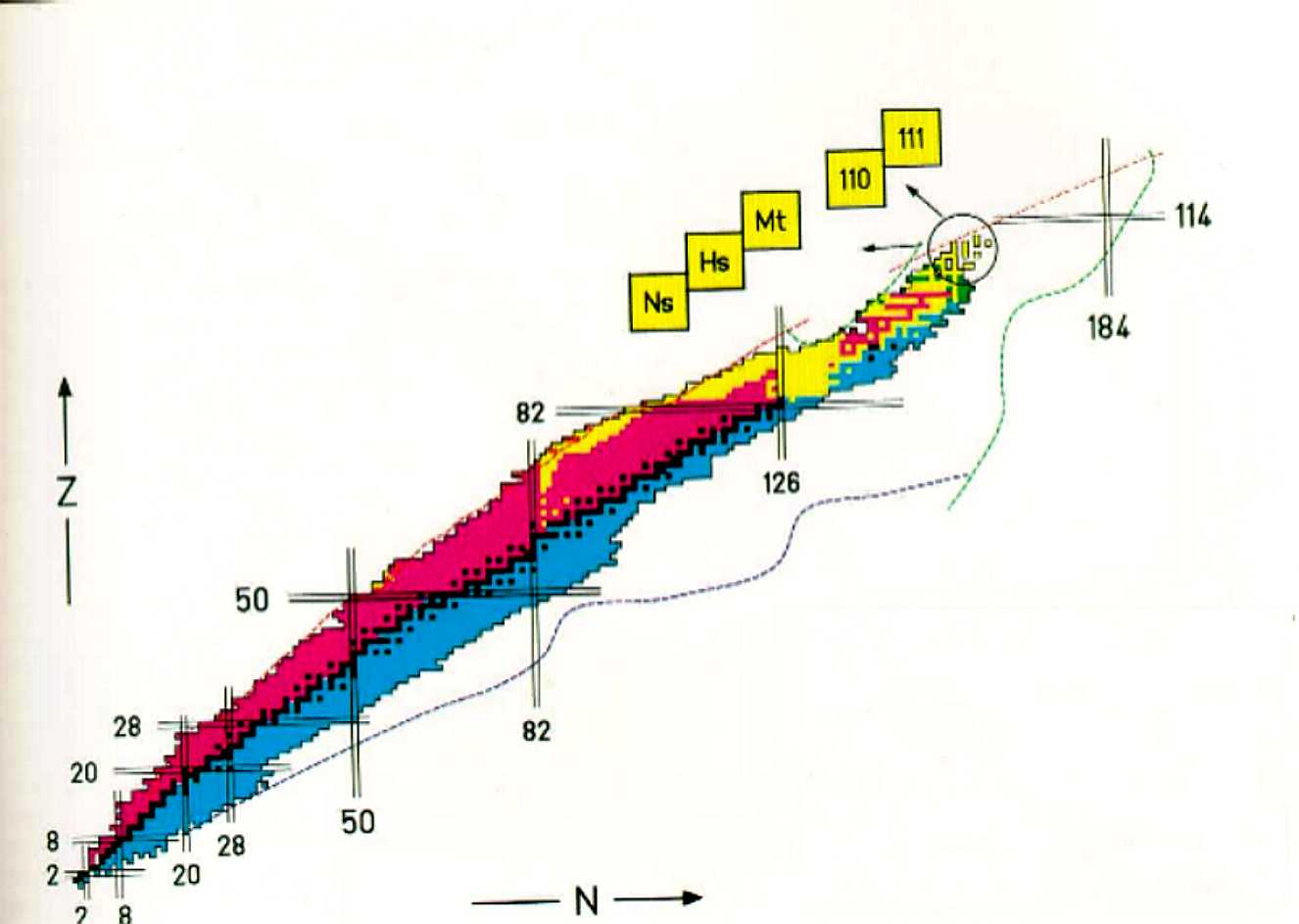
identified as a "magic" number. Figure 2 and 3 refers to the stability
of nuclei. The pennisula illustrates known nuclei and an island of
superheavy nuclei that is relatively stable in the sea of instability:
Common examples of this occurrence include the double magic
nuclei 4He, 16O, 40Ca, and 48Ca,
as well as 208Pb. In these nuclei, both the protons
and neutrons form filled shells, so that these nuclei all have particularly
high binding energies.

The magic numbers were successfully explained
by the nuclear shell model developed in 1948. It deals with the local stabilization
of certain proton and neutron configurations brought about by quantum mechanical
effects. It took another twenty years to resolve the conflict that arose
between the shell model and the classical liquid-drop model. One such issue,
according to the liquid-drop model, involved the belief that repulsive
electrostatic forces would prevail around element 110 and effectively terminate
the periodic table of the elements because heavier nuclei, if formed, should
disintegrate into two fragments (Sea 90). The
shell model suggested that it should overcome the electrostatic forces
and even stabilize heavier elements. Three theoretical papers were presented
in the late l960’s : by V.M. Strutinsky, who combined shell- and liquid-drop
models; by W.D. Myers and W.J. Swiatecki, who predicted strong shell effects
beyond the existing elements; and by H. Meldner, who concluded that 114
should be the next "magic" proton number after 82 (Wal
92).
Further theoretical studies predicted
a whole superheavy "island of stability" around proton number Z=114 and
neutron N=184. This was well separated from the "mainland" of known nuclei
by the "sea of instability." Element 114 of atomic weight 298 (Z + N) became
the Holy Grail for nuclear chemists and physicists.
In the late sixties, it was recommended
that a universal heavy ion accelerator be constructed. Such an accelerator
would allow the study of all nuclear reaction that could be involved in
the production of SHE. In principle, the method of producing the nuclei
is always the same: a beam of accelerated particle from the linear accelerator,
or the heavy ion synchrotron, is directed against a foil or a piece of
matter, referred to as the target. Nuclear reaction in the target produce
exotic nuclei which are then separated by separators according to nuclear
charge and mass.
The universal heavy ion accelerator
was only the first step in achieving heavy element synthesis. With its
chain of individual resonators , it was now possible to change the energy
of the ions by small increments and set the ion energies in a reproducible
fashion (Gru 97).
The second key involved the velocity
filter SHIP (Separator for Heavy Ion Products). SHIP had the task of filtering
out the rare fusion products. This process was extremely difficult. It
meant detecting one superheavy nucleus per day from the flood of more than
three thousand billion beam particles and reaction products incident on
the filter every second. Figure 4 displays a schematic diagram of the velocity
filter SHIP (Mun 81).

The particle beam coming from the universal
heavy ion accelerator is on thin layers of lead or bismuth, which form
the outer circumference of the target wheel. The target wheel itself is
rapidly rotated to avoid overheating. The target wheel makes is possible
to irradiate lead or bismuth metal foils with high currents of particle
beams despite their relatively low melting points. Eight target now rotate
with 1,125 rpm through the beam.
The reaction products are identified
by a sophisticated detector system which registers the time and the position,
measured vertically and horizontally, at which time an ion passing through
SHIP is implanted in the detector.
The fate of the initially implanted
new element can be followed step by step through several generations of
known radioactive decay products. Each such decay chain has it own fingerprint
characterized by the lifetimes of the isotopes and the energies of the
particles emitted.
On November 9, 1994, a German team
at Darmstadt beat out the American team in Berkely and the Russian team
in Dubna with the creation of element 110. The Germans bombarded a 208Pb
target with a beam of 62Ni ions. Four atoms of element 110 with
a mass number of 269 were observed. During the subsequent week, isotopes
of element 110 were also created. Then on December 8, 1994, the first element
of 111 was created using 64Ni projectiles and a 209Bi
target. The mass number of the produced isotope was 272. In addition to
the Germans discovery of element 111, they observed that the decay chains
of 272111 produced new isotopes elements 109 and 107. Figure
5 shows three representative decay chains of the new elements with a
-decay energies and lifetime of the chain links indicated (Gru
97).

On the Road to Element 114
With researchers recent
successes , the number of known SHE have nearly doubled. These experiments
suggest there are even heavier elements waiting to be discovered, including
the "magic" element 114. The whole landscape presented in (figure 6) from
Pb to the superheavy elements represents a contour map of the stabilizing
shell energy.
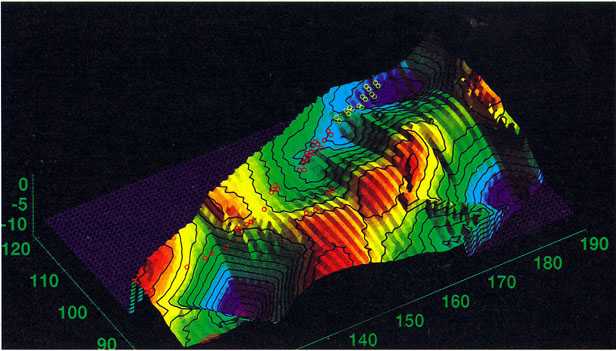
It is from this map that predictions can be made beyond element
111. In particular elements 112 and 113 should suffer from destabilization.
Heavier isotopes of these two elements would already profit from the superheavy
island, but they can not be made with stable isotope targets and projectiles
(Gru 97). Element 114 and 115 are already in
the shore region of the island of stability, but element 116 will be close
to its center (Wal 97). This indicates a more
stable chain path may be found with element 116.
Similar decay properties are calculated
for the three succeeding a -decays through elements
114Þ 112Þ
110 down to elements 108 and 106. The chain probably ends at element 104
by spontaneous fission of 266104 (Gru
97). All nuclei of this decay chain are currently unknown, but the
half lives and decay properties are unique for the decay of superheavy
elements.
The road to SHE development began
nearly 60 years ago with the first human made elements. This research has
taken us on many paths and in many directions. Some discoveries have had
grave consequences for humanity. For example, the production and
use of plutonium in nuclear weapons, while other discoveries have permitted
us to theorize about the unknown, not for profit or weapons but just for
a greater understanding. This purity of thought is akin to why humanity
attempts difficult pursuits, because they can.
BIBLIOGRAPHY
(Atk 91) Atkins, P.W. Quanta: A Handbook of concepts, 2nd ed.
Oxford: Oxford University Press; 1991. 579 p.
(Atk 95) Atkins, P. W. The Periodic Kingdom. New York: Basic
Books;1995. 161 p..
(Bou 89)Bourne, J. An Application-Oriented Periodic Table of the Elements.
Journal of Chemical Education 1989 Feb; 66: 28-39.
(Cox 89) Cox, P.A. The Elements: Their Origin, Abundance, and
Distribution. Oxford: Oxford University Press; 1989. 234 p.
(Emp 91) Employ, J. The Elements, 2nd ed. Oxford: Oxford
University Press; 1991. 356p.
(Gru 97) Gruen, D. Elements by Design. Oxford: Oxford University
Press; 1997. 420 p.
(Jac 96) Jackson, A. Chemistry, 3rd ed. New York: Wiley; 1996.
1335 p.
(Mas 88) Mason, J. Periodic Contractions Among the Elements:
Or Being the Right Size. Journal of Chemical Education 1988; 65:
17-20.
(Mun 91) Munzenburg, et al. Z Physics 1991 July; A302, 7
(Sea 63) Seaborg, Glenn T. Man-Made Transuranium Elements.
Englewoord, NJ: Prentice-Hall; 1963. 120 p.
(Sea 90) Seaborg Glenn T, Loveland, Walter P. The Elements Beyond Uranium.
New York: Wiley; 1990. 359 p.
(Wal 97) Walter, P. The Transuranium Elements. New York:
Wiley; 1997. 370 p.
(Zin 79) Zinder, F. Formation of the Chemical Elements.
Oxford: Oxford University Press; 1979. 426 p.
Updated:
May 25, 1998
Finis







