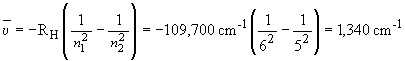
Problems from Kotz & Treichel
35a) Six emission lines are possible. These are:
n = 4 ® n = 3; n = 4 ® n = 2; n = 4 ® n = 1n = 3 ® n = 2; n = 3 ® n = 1
n = 2 ® n = 1
b) The n = 4 ® n =
1 transition will have the highest energy.
c) The n = 4 ® n = 3 transition
will have the longest wavelength.
41) The lowest energy line in the Pfund series corresponds to the n
= 6 ® n = 5 transition. The frequency
(in cm-1) is given by:
Using RH = 109,700 cm-1,
43) The wavelength of the electrons in the beam is given by:
45) The wavelength associated with the bullet is:
73) The shortest wavelength an excited H atom can emit arises from
an
n = ¥ ®n
= 1 transition. The wavelength (in nm) would be:
Additional Problems:
1) The wavelength of an n = 6 ®n = 5 transition for He+ is:
An example of a transition that would yield a photon of visible
light would be an n = 6 ® n
= 4, which would emit 656 nm light (red).
2) The ionization energy of a hydrogen atom with a principal quantum
number of five is: