Fuel Cells for Today's Energy Needs By Galen Schmitt The current state of power production, from hydroelectric to fossil combustion, to nuclear power production have many people questioning the long term ability of the human population to supply enough power to maintain our advanced technological society. While a combination of conservation, efficient production of power and alternative fuel sources are the key, it is imperative that we use technology, which has been proven to work because we have to face the reality that we are in an advanced, technology society, where many people are reliant on power, supplies and food from locations other than where we live. Fuel cell technology was first pondered in the mid 1800's. One would think that almost 150 years would be enough time for a concept of such relevance to have blossomed into a working method, but the feasibility of people creating their own energy source has never caught on in the mainstream of today's society. Fuel cells may have been invented in 1839 but have not been widely applied because the technology is demanding, and because other technologies have been dominating the marketplace. However, fuel cells exceptional environmental performance and their outstanding efficiencies have recently caused renewed interest, buoyed by advances in engineering and material science. Fuel cells may today provide a real alternative for a cleaner way of handling power. The only time people seem to conserve and strive for efficiency is when dictated to do so by the government such as the victory gardens and mandatory power outs of the world wars. This is partially due to lack of people studying the science of fuel cell technology, and then explaining what they know to other people. The majority of research being done in the field is done in esoteric laboratories, not in readily accessible locations. There are a few positive things, which have been done to promote fuel cells. Beginning in 1996, acknowledging that fuel cells can fill the gap between energy supply and rapidly increasing demand, the federal Bonneville Power Administration, which supplies power to most of the NW, began working with IdaTech, then known as NW Power Systems to encourage development of a compact, clean and efficient fuel cell power plant that could be placed at the home. They purchased the first 50 units which run in MT and OR. (1) The current west coast power crunch, which has raised power rates throughout the country and posses threats to environmental protection and air quality, such as the lifting of NOX restrictions in Tacoma recently, should be seen as a wake up call to people for the necessity in large scale development of alternative energy production. "Energy conversion via fuel cells represents one of the best ways to achieve efficient conservation of remaining fossil fuels, because it is possible to obtain more work and less pollution from a dollars worth of fuel with a fuel cell than with any other device."(2)
Fuel Cell Chemistry. All fuel cells consist of four basic components, which are catalysts (sometimes referred to as a Polymer Electrolyte Membrane), fuels (H from various hydrocarbons), electrolytes, and electrodes. The crux of research done to date is finding the correct combination of components, leading to maximum efficiency within the cell. The simplified explanation of the driving force behind the theory is, potential energy in fuel (in the form of kJ) serves as chemical energy, which enters the cell apparatus and electrical power (in the form of kWh) exits after the reaction of H2 from the fuel and O2 from the air. The O2 and H2 travel along a pathway similar to the electron flow in a battery (Figure 1). The H2 fuel is necessarily desulfurized to avoid inhibitive reactions on the anode, then mixed with steam and transformed to 2 H atoms, which react to produce electrical power. FCs conduct oxygen ions produced by oxygen reduction at the cathode. The electrochemical potential of the cell follows the Nernst equation, E*cell=RT/nF x ln of (activity at cathode)/(O activity at anode). The free energy potential is explained by Delta G=-number of electrons (n) x Faraday's constant (96500col/mol e-) x E*cell. When methane is used, CH4+2O2->CO2+2H2O(l), this can also be calculated for emf (electromagnetic frequency), which allows for calculation of free energy values. Emf=E*cell=-Delta G/(n x F) (see Table 1 for half cell equations and free energy). Under fuel cell conditions this usually equates to ~ -700+/-, which indicates heat is created (3). The formed H2O is produced at the anode via electrochemical reactions of oxygen with hydrogen and exchange of electrons on the electrodes. The other common waste product is CO, which is used in an internal coolant. Unlike regular combustion reactions; fuel cells do not suffer from the Carnot cycle limitation that decreases efficiency in combustion system and is explained by the equation: [absolute temperature entering]-[absolute temperature exiting]/[absolute temp. entering]. (3) One of the problems of research of fuel cell thermodynamics and kinetics is that they do not generally operate under completely reproducible conditions during experimentation. The fuels involved can exist either in gaseous, liquid or solid state and at a wide variety of temperatures and pressures. Free Energy Calculations (4) H(kJ/mole) G (kJ/mole) Methane (CH4) -78.81 -50.75 Hydrogen 0 0 Oxygen 0 0 H2O (l) -285.83 -237.18 H2O(g) -241.82 -228.59 Carbon monoxide -110.52 -137.15 Carbon dioxide -393.51 -394.36 CH4+H20 (g)->CO+3H2 210.11 142.19 CO+1/2O2->CO2 -282.99 -257.21 3H2+2O2->CO2+2H2O(l) -956.17 -868.72 3H2+2O2->CO2+2H2O(g) -877.15 -851.54 CH4+2O2->CO2+2H2O -886.36 -817.97
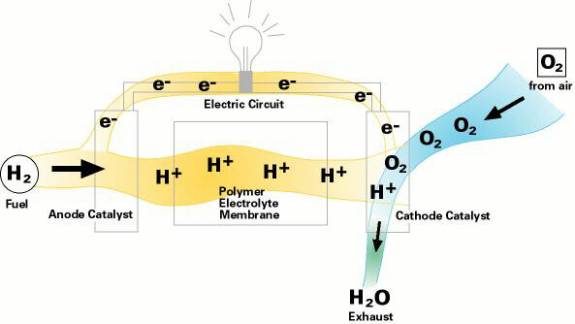
Figure 1. Fuel Cell Pathway
Fuel Cell Economics and Benefits In addition to the states of fuels, there are several other inhibitive problems, which are holding back the use of fuel cells such as the Solid Oxide Fuel Cell. Economic feasibility and the widespread use and advancement of fuel cells depend on the start up costs. The creation of fuel cells will continue to be expensive until technology is pushed to create cheaper catalysts and electrodes, which are the costliest components. Fuel cells, with their decreased emissions of NO2, SO2, and CO (compared to combustion energy), are likely to lead to the adoption of fuel cell technology, if environmental quality and regulations are of concern. Siemens-Westinghouse Power Corporation in Pittsburgh, PA, claims NOX levels at <. 5ppm, SOX, CO and VOC levels at 0 ppm (5). IdaTech's methanol fueled fuel cells are releasing <5ppm CO2, <1ppm CO and no measurable NOX. A very high proportion of the cost of supplying electrical power by conventional means to consumers is the cost of transportation; this can be as high as 60% of the total costs from the construction and maintenance of transmission lines. Thermal energy from fuel cells cannot be piped over long distances so localized fuel cells are the ideal setup. Satellite "low power" fuel cell power plants are a solution to the rising cost of power produce from the combustion of fossil fuels and other organic petroleum products. Fuel cells show potential applications in remote and emergency power in locations, which might exist off the grid, as portable and propulsion energy providers and as utility power reserves in populated areas. Unlike gas combustion systems, SOFC's do not require a cooling system, as they are cooled internally from the reforming action of the fuels. The oxygen and hydrogen based fuel development shows the highest efficiency and lowest fuel costs of economic value as replacements in urban areas and environmentally sensitive areas compared to photovoltaic and wind power generators. Hydrogen/oxygen based fuel cells require less maintenance and are therefore applicable to remote locations. The lack of maintenance and reliability is a rational alternative to large wind powered turbines, which can negatively affect wildlife, require travel to them, and can lead to confrontation with environmentalists. Fuel cells have the capability of promoting themselves as they provide an alternative to the trading of emission stocks by large corporations and industrial stockholders. The resource conservation and environmental quality benefits of fuel cell advancement and implementation are quite numerous. Studies done suggest the largest economically feasible reasons; as opposed to environmentally based decisions; for fuel cells is as a feasible petroleum product alternative. Fuel cell power systems could exceed $3 billion in the next few years, promoting the economic strategies, which are primary concern to the current governmental bureaucrats. Mass transit power could be supplied by fuel cells such as those already created and marketed by Ballard. Recent studies also suggest projected demand for transportation fuel cells in 2007 could reach $9 billion. Fuel cell technology is a means to provide energy security by decreasing reliance on oil imports, which fuel so many of the tensions between developing nations and technological societies such as ours. Estimates predict that if 20% of cars used fuel cells, oil imports could be reduced by 1.5 million barrels/day. 10,000 fuel cells running on non-petroleum fuel, such as methane, would reduce consumption by 6.98 million gallons/year. The US Department of Energy projects if 10% of automobiles were fuel cell powered, particulate matter and regulated air pollutants would be cut by 1 million tons/year and 60 million tons of greenhouse gas altering CO2 would be eliminated. (5) According to a Utah based energy firm a 25% replacement of gas turbines by SOFCs would result in a yearly fuel savings of $250,000,000. Even large petroleum extraction companies see the potential of alternative energy sources, and the potential to wean our society away from petroleum reliance. "The days of the traditional oil company are numbered, in part because of emerging technologies such as fuel cells for transportation and countries with emerging economies that will exert more control over their natural resources." Peter I. Bijur, chairman and CEO, Texaco Inc. The fuel cells can also promote economic growth. They could create new markets for steel, electronics, electrical, chemical, engineering and control industries. Fuel cells could create tens of thousands of high quality, people-empowering jobs. A.D. Little, Inc. projects fuel cells will reach $3 billion with a market of 1500-5000 jobs. If just 20% of the cars were equipped with fuel cells 800000 jobs would be created. Solid Oxide Fuel Cells. Table 2. Fuels Efficiency Power Op. temp H2,CO,CH4 45-55%+ cogeneration 5 kW/m² 700°C One highly promising fuel cell, the Solid Oxide Fuel Cell (SOFC) could be used in big, high-power applications including industrial and large-scale central electricity generating stations. Some developers also see solid oxide use in motor vehicles and are developing fuel cell auxiliary power units, which are small mobile devices with SOFCs. A solid oxide system usually uses a hard ceramic material instead of a liquid electrolyte (coined a Polymer Electrolyte Membrane), allowing operating temperatures to reach 1,800 degrees F. Power generating efficiencies could reach 60% (some studies predict up to 70%).>
Design and Structure One type of SOFC uses an array of meter-long tubes, and other variations include a compressed disc that resembles the top of a soup can. Planar and tubular SOFCs stack fuel cells in an electrical series through interconnections to produce practical amounts of power. In a planar design, fuel and air flow across opposite sides of thin, flat cells. In the tubular design, fuel and air flow on the outside and inside of closed-end, cylindrical cells. Planar designs have advantages in power density and compactness, and tubular designs have strong reliability advantages. A Westinghouse tubular cell unit has completed almost eight years of continuous operation-the longest running fuel cell of any type. Tubular SOFC designs are closer to commercialization and are being produced by several companies around the world. Siemens-Westinghouse, is one of the leaders in SOFC design and technology. The tubular designs they have created operate at lower temperatures and the materials do not cost as much. Demonstrations of tubular SOFC technology have produced as much as 220 kW. Allied Signal Aerospace Company has developed a SOFC that resembles a monolith similar to a corrugated cardboard box. (Figure2) . The active cell components (cell membranes) serve as the passageway for the fuel and oxidant streams. Modern SOFC's have simpler chemistry and electrochemistry, compared to older models, but are less advantageous thermodynamically, but they can lead to 100% H utilization compared to 50% in typical energy production. The electrolyte is often composed of yttrium-stabilized zirconium. The anode consists of metallic nickel and yttrium-stabilized zirconium, with the cathode made of strontium-lanthanum manganite.
Figure 2-too difficult to download.
|
e |
|||
|
|
|
|
|
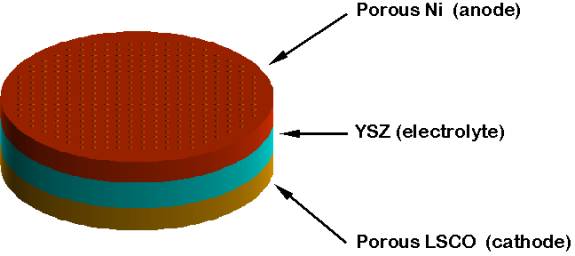
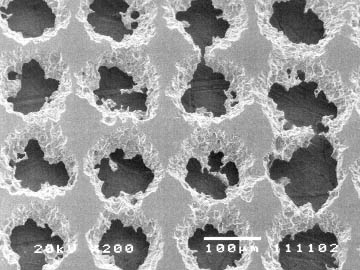
Figure 3 SEM image of etched nickel foil. Ragged hole edges improve efficiency by increasing surface area.
SOFC's have been developed, which use ceramic solid Zirconium. This structure helps correct the problems of stacking and sealing. Under high temperatures such as those in SOFC's cracking can occur, but the combination of stacked Zirconium plates separated by thin, porous layers of ceramic felt helped to ease the high temperature strain. Estimated costs for SOFC's are $200/kW based on the cost of bulk oxides for the electrodes. Problems, and potential employment opportunities for solutions, arises from preparation of compounds with the correct purity, rather then the cost of components themselves. One potential application of the technology, based on the fact that efficiency of fuel cells is independent of size, is the development of Micro-SOFC's (see Figure 5), which could potentially be used in small vehicles. This apparatus is the simplest system yet devised for the direst use of hydrocarbons in a fuel cell device. The idea is a small battery, which does not require a recharge.
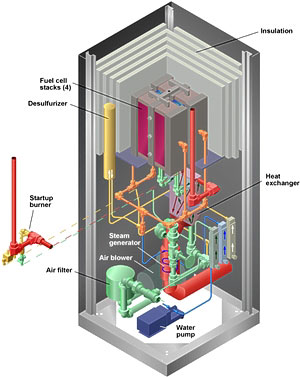
Figure 4a McDermott Technology's Diagram of a planar SOFC
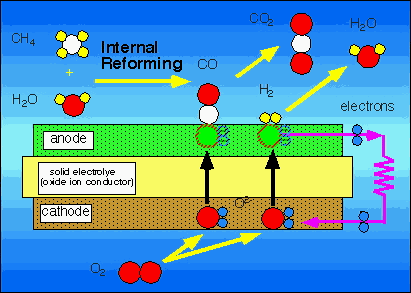
Figure 4b Schematic of a Planar Solid Oxide Fuel Cell
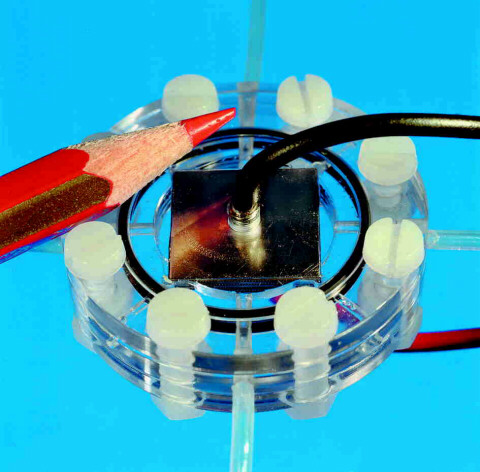
Figure 5 Micro Fuel Cell developed by Fraunhofer ISE
Fuel Sources SOFC's run on the basis of hydrogen as the fuel source. A fuel cell system initially reforms natural gas fuel into more reactive fuels such as hydrogen gas (H2) and carbon monoxide (CO) by combining the C and O from CH4 and H2O, respectively, at the anode and combining the H2 with O from O2 from the cathode (See 4b). The fuel cell then converts chemical energy into power by electrochemically combining the reformed products and air across a dense, ion-conducting electrolyte layer sandwiched between porous fuel and air electrodes (6). The Pacific Northwest cannot rely on photovoltaic cells, but can on natural gas and waste products from agriculture such as methane from animal and human waste. Technology exists and is being researched to use more complex hydrocarbons such as pentane. Hydrocarbons are non-polar, so there is not the problem of compounds resinating on the internal structures, which leads to the need for maintenance, and decreases efficiency in fuel cells as experienced in combustion systems. This also has implications in a method to reuse spent or polluted organics, which may have been used, spilled or otherwise wasted. CH4, as the fuel source, favors devices that operate at high temperatures such as SOFCs exhibit. The combustion of methane is expressed as CH4+2O2-CO2+2H2O. Fuel cells electrochemically oxidize mixtures of H and CO, but they require a "reformer" to formulate non-H fuels into usable H. They use anodes with nickel to catalyze CH4-H2+CO2 (Figure 3). There is research on formulations of anodes to increase efficiency by recuperating and using waste heat to supply the endothermic reaction, in essence making the cell a self-contained mechanism. A simple 25kW fuel cell from Siemens-Westinghouse, which runs on both diesel and JP-B fuels, has shown definite promise for co-generation fuel cells. SOFC's have a large tolerance to impurities in the reactant gases compared to IdaTech's methanol fuel cell, which failed due to impurities in the fuel source. SOFC's can also use exhaust "waste" heat to continue the cell reactions. (6) The principle of CHP, or combined heat and power, also known as cogeneration, has lead to the study of integrating biomass or coal gasification into fuel cell power production. The idea of fuel cells combined with gas turbines has shown potential of creating a very efficient power system. (Figure 6)
Conclusion Efficient and "green" products, which promote sustainable living, have gained prominence and a place in the scientific and economic disciplines of today's society. Unfortunately, some products require such a stray from typical technology that they are not promoted. Some fledgling technologies require a push from people to develop technology to make these advances affordable. Fuels cells, especially complex ones such as Solid Oxide Fuel Cells, have the potential to lead the country to a more reliable way to meet our growing power needs. The acceptance of new technology requires the science community to study, and then explain the underlying concepts to the public and other professionals. This is the first step towards getting people to pursue the government to step up the creation; they already have some branches of government working on fuel cell research; and development of technology to bring fuel cell power into everyday reality and bring them into schools, businesses, and homes.
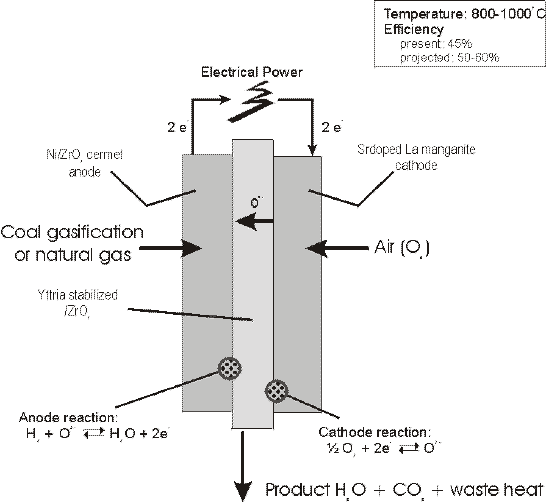
Figure 6. Cogeneration fuel cell
|
|
|
|
|
|
|
|
Animation of IdaTech's Fuel Cell
Technology |
Check out this site
Works Cited and References 1) www.idatech.com 2). Fuel Cells for Public Utility and Industrial Power. R Noyes editor. 1977. Noyes Data Corp.NJ 3). Symposium Proceedings. Institute of Gas Technology. Chicago. 1981. 4). Free Energy information supplied by Dr. Clyde Barlow. The Evergreen State College. 5). Fuel Cells. NY. Academic Press. 1963. Editor Raymond F. Baddour. Background information on thermodynamics and kinetics of fuel cells. 6). w4.siemens.com 7). The Role of Fuel Cell Technology in the International Power Equipment Market. A.D. Little, Inc. Cambridge, MA. 9/93. 8). Fuel Cell Systems 2, 5th Biennial Fuel Cells Symposium of the ACS. 1969. ACS Publications. 9). www.gri.org 10). Figure 4a from www.mtiresearch.com References Fuel Cells. Angus McDougall. 1976. Halsted Press. www.fuelcells.org, www.mat.bnam.ac.uk, www.worldwatch.org www.humboldt1.com/~michael.welch/extras/homefuelcells.pdf