Environmental Analysis: Hydrologic Projects at The Evergreen State College
Piezometer Wells
By:
Gail A. Fullerton
Gavin Glore
Nadia Madden
Aspen Madrone
David Short
Abstract
This study adds baseline water chemistry analysis and additional hydrologic data to existing studies. The three sites on The Evergreen State College (TESC) campus: an alder forest, a wetland and a paved area with EPA monitoring wells. The study also includes maps to help clarify the location of existing piezometers and a geological description of the peninsular area where TESC is located. During winter quarter 2001 students from the Environmental Analysis program at (TESC) monitored the static elevation of water in existing piezometers, and analyzed water samples taken from each site for anion concentration, dissolved oxygen, alkalinity, conductivity and pH. Both new and existing data were incorporated into this report.
Introduction
A number of hydrological studies have been conducted on the campus of the Evergreen State College. A number of piezometers have been installed in different areas on campus. These piezometers have been used to study ground water. In the Environmental Analysis program, students have analyzed the water chemistry of the piezometers and the monitoring wells. To date, there have been few comprehensive efforts to group the results of these studies together. In our study, we continued to monitor water depths and chemistry of these piezometers. We also mapped the locations of the wells on a GIS map of the Evergreen campus; linking each to the various spreadsheet data we had access to. The purpose of this document is to serve as a compilation of references that can be used as a resource for future studies characterizing the groundwater hydrology of TESC.
Site Descriptions
General geology and soil
The TESC campus is located in the
peninsular area of Thurston County.
From top down the soil and geology of the area is characterized by Noble
and Wallace 1966. The top layer is
Vashon till both subglacial and ablation.
Subglacial consists of unsorted gravel in a matrix of sandy silt and
clay, commonly called hardpan. Ablation
till, brown snady and gravelly soil accompanied by a few boulders. This is underlain by Covos sand and Vashon
advance outwash sand. Covos sand can be
as thick as 300 ft. in Thurston County it is seen in the banks on the sound in
west Olympia as thick as 80 ft. Colvos
sand has a salt and pepper appearance. The next layer down is the Kitsap
formation, a fine-grained unconsolidated deposit composed of clay and silty
sand with some sand. It can also have
peat layers. It often has gravel lenses
at its base. Below this is Vashon
drift or Salmon Springs drift. The two
differ in ground water potential but their physical differences are
subtle. Salmon springs drift turns to
a red color where expose due to iron oxides.
It is generally yellowish brown to reddish brown. It is found 3 to 30 feet above beach
level. Pre Salmon spring deposits occur
mostly at beach level. They are mostly
slity clay. This clay is often a dark
blue gray color but may also be brown gray or buff.
Wetlands
This area south of Evergreen Parkway (see Map 1) was converted to agricultural use before becoming the property of The Evergreen State College (TESC). It is thought to have been a wetland before this conversion and still retains wetland characteristics. Pacific Crab Apple, Oregon Ash, Douglas Fir, Western Red Cedar and Willow trees can all be found here. There are patches of sedge and canary grass. Most of the area is thick with underbrush such as Hardhack and Scotch broom, and the presence of these invasive non-native pioneer species suggests disturbance Soil logs from the wetland area indicate the presence of pre-salmon springs clay and the Kitsap formation. The area is underlain by at least one confined aquifer. Soil logs suggest the presence of several perched aquifers.
Motor Pool
Four monitoring wells (see Map 2) were installed in the TESC motor pool parking lot following a fuel spill. These wells are used to monitor the aquifer under the area for possible contaminants. The aquifer being monitored is believed to be the same aquifer underlying the wetland.
BC Parking lot
This area is located between the B and C parking lots (see Map 3) and the Evergreen Parkway. The first four piezometers are located along Huston Creek. Huston Creek is the destination of most of the runoff water from B and C lots. Eight additional piezometers were installed in the fall of 2000. The forest types in this area are Douglas Fir/Western Hemlock, Alder and Big Leaf Maple. The wells are screened at different depths, so it is possible that they were used to study different aquifers.
Background
Wetlands
The most comprehensive study on the wetlands site was a hydrological study done in the spring of 2000 by Eli Callison and Dan Smith. Piezometer wells were installed on transects placed at 100 ft intervals along Evergreen Parkway. These wells were monitored regularly for water depth. Hydraulic conductivity of the aquifer was determined from a slug test. The most important results of their report were:
· Characterizing the groundwater as having a NE flow.
· Minimal infiltration (due to confining layer) near the surface
· Perched aquifer conditions
The study hypothesized that the wetlands may be a recharge area for the shop wells at the motor pool.
A study was also done to evaluate the feasibility of a proposed wetlands area in the southeastern corner of campus. This group made a GIS map of the proposed wetlands site, including the locations of piezometers and vegetation types. They also digitized the area they deemed most suitable for wetlands creation.
Motor Pool
TESC contracted Creative Environmental Technologies (Tacoma, WA) to conduct a groundwater characterization study in 1999 at the campus motor pool, where leaking petroleum tanks had been removed in 1993. The study was to determine whether the site had been fully remediated for petroleum contamination. In this study four monitoring wells were installed in the lot. Transglobal Environmental Sciences (Lacey, WA) performed a detailed soil chemistry analysis for petroleum contaminants. According to the monitoring well data, groundwater in the motor pool area appears to flow to the northwest. However, gasoline contaminants were previously found to have traveled in a southeasterly direction in a 1995 study by Benz. It is believed that the contaminants were traveling in a seasonal perched aquifer. Ongoing water elevation monitoring is conducted by Evergreen Facilities (Contact Richard Davis, Facilities Engineer).
B
lot
In the spring of 2000, four piezometers were installed near Houston creek, under an alder forest adjacent to parking lot B. The focus of this project by Spike Minczeski was to measure the hydrology of the water table for the aquifer and Houston creek. Water elevation data for the four piezometers is available from February to May of 2000. Other pertinent data is the soil logs compiled from the piezometer excavation. In the fall of 2000, environmental analysis students took the GPS points of the existing piezometers and dug eight new piezometers perpendicular to Houston creek.
Methods
Water Sample Collection
Water was collected from all of the piezometers in the same manner. First water levels were taken with the Solinst water depth meter. The monitoring wells water level elevation was completed in strict ordinance with the instructions from Dr. Stroh (See Appendix 1). Then water was bailed into either 500 mL glass DO bottles or/and 500 mL plastic sample bottles. Water temperature was taken from the plastic sample bottle for one minute.
Dissolved Oxygen
For dissolved oxygen the HACHS Winkler titration kit was used to fix the sample in the field with the addition of manganese and alkaline iodide pillow packet. Then back at the lab the fixed sample is acidified with sulfuric acid packet and mixed. At this point the floc must be allowed to settle out half way down, then remixed and allowed to resettle before titrating 100 mL of sample in beaker on stir plate with thiosulfate solution. When solution reached a pale yellow color six drops of starch were added to turn the solution dark blue. Thiosulfate solution was added until the solution became clear. Three titrations were completed on each sample.
pH
In the lab pH was measured with the Sentron Argus pH meter. The meter was calibrated with pH 10 solution and pH 4.01 solution, both at 25 degrees C.
Conductivity
Conductivity was measured in the lab with a meter by HACH, Serial # 1192. It was calibrated with a NaCl standard.
Anion Concentration
A Dionex 2020I Ion Chromatograph was used to analyze samples for anion concentrations by comparison from standards of nitrate, nitrite, phosphorous, chloride and sulfate. Standards were made from stock solution made following the modified EPA method 9056A (Appendix B).
Results
Piezometer Elevations
Water elevation data was acquired for parking lot B and for the Motor Pool piezometers. The static elevations were plotted along with previous data (from the two previous B lot studies and from Evergreen Facilities Motor Pool data) to compare elevations over time. While water depths were also measured in the wetlands, the data was insufficient for comparison with previous studies and is not shown here.
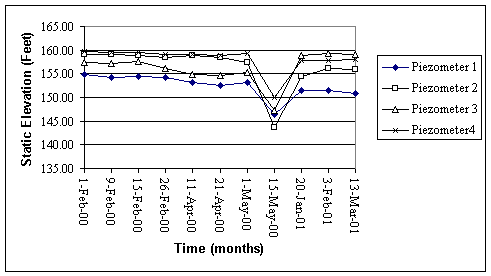
Figure 1. Static elevation of water
in B lot piezometers from 2/01/00 to 3/13/01.
All elevations are given as feet above mean sea level.
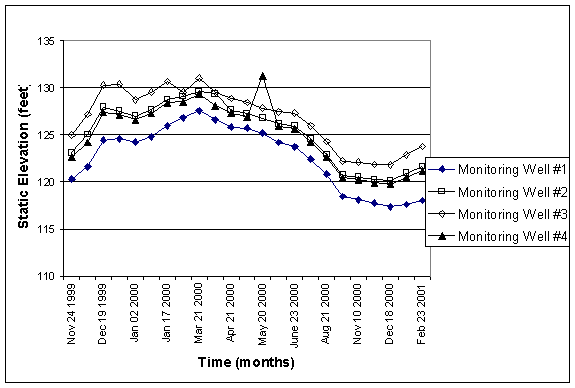
Figure 2. Static elevation of water
in Motor Pool monitoring wells from 11.99-2.01. All elevations are given as feet above mean sea level.
Figure 2 represents the static elevation of water table at the motor pool monitoring wells from 11/1999 to 02/2001. Notice how the elevation increased over the winter of 1999, while only a slight increase occurred in the winter of 2000.
|
Month/Year |
Rainfall
Total (mm) |
MW
# 1 |
MW
# 2 |
MW
# 3 |
MW
# 4 |
MW
Avg. |
|
11.1999 |
311.15 |
120.27 |
123.10 |
124.96 |
122.64 |
122.74 |
|
12.1999 |
316.48 |
123.53 |
126.82 |
129.27 |
126.21 |
126.46 |
|
1.2000 |
193.29 |
124.97 |
127.79 |
129.62 |
127.41 |
127.45 |
|
2.2000 |
168.66 |
126.81 |
129.06 |
129.51 |
128.51 |
128.47 |
|
11.2000 |
85.34 |
117.93 |
120.37 |
121.96 |
120.05 |
120.08 |
|
12.2000 |
111.76 |
117.36 |
120.11 |
121.82 |
119.76 |
119.76 |
|
1.2001 |
88.39 |
117.61 |
120.92 |
122.86 |
120.48 |
120.47 |
|
2.2001 |
68.33 |
118.01 |
121.62 |
123.75 |
121.16 |
121.14 |
|
% change 11 |
-72.57% |
-1.95% |
-2.22% |
-2.40% |
-2.11% |
-2.17% |
|
% change 12 |
-64.69% |
-5.00% |
-5.29% |
-5.76% |
-5.11% |
-5.29% |
|
% change 1 |
-54.27% |
-5.89% |
-5.37% |
-5.22% |
-5.44% |
-5.48% |
|
% change 2 |
-59.49% |
-6.94% |
-5.76% |
-4.45% |
-5.72% |
-5.72% |
|
Avg. % change |
-62.75% |
-4.94% |
-4.66% |
-4.46% |
-4.59% |
-4.66% |
|
Table 1 - This table compares the
total monthly rainfall (mm) at TESC and
the average |
||||||
|
static
H20 elevation (feet) in the Motor Pool monitoring wells from two consecutive
years. |
||||||
Table 1 analyzes the changes in rainfall and piezometer elevation between the winter of 1999-2000 and 2000-2001. The % change represents the monthly relative change. All four months examined exhibited large negative changes in rainfall, where rainfall decreased over the course of the month. Absolute change ranged from –100 (Feb) to –225 (Nov) mm with an average absolute change of -159 mm. The relative change ranged from a 54% decrease (Jan) to a 73% decrease (Feb) with an average decrease of 63%.
Static elevation of H20 in the monitoring wells also showed a negative correlation with time (Figure 2). Monitoring wells exhibited absolute water elevation changes of between –2.35 ft and –8.80ft, with an average absolute change of –5.92ft. Relative change in static water elevation also negative, but the % change was much less than that of the rainfall totals ( Avg. % change rainfall = -62.75 mm, Avg. % change water elevation = 4.66 ft, Table 1). It is interesting to note that November has the highest % change for rainfall totals, but the lowest average % change for monitoring well water elevation.
Ground Water Chemistry
|
pH |
conductivity
(umhos) |
alkalinity
(mg/L) |
|
5.81 |
325 |
52.836 |
Table 2: Field
measurements of Motor Pool monitoring wells on 3/09/2001.
|
Piezometer |
pH |
conductivity
(umhos) |
alkalinity
(mg/L) |
|
P1 |
6.6 |
275 |
40.06 |
|
P2 |
6.1 |
100 |
12.43 |
|
P3 |
6.55 |
300 |
55.94 |
|
P4 |
6.7 |
310 |
43.51 |
Table 3: Field measurements and alkalinity of Houston Creek (B-lot) Piezometers on 1/20/2001
|
Piezometer |
pH |
temp. deg. C |
conductivity
(umhos) |
Winkler
DO (mg/L) |
Stn.Dev DO |
|
3 |
6.42 |
8 C |
160 |
11.4 |
0.208 |
|
Manhole |
6.72 |
8 C |
880 |
7.56 |
0.061 |
|
2b |
5.65 |
6 C |
230 |
9.18 |
- |
|
2a |
6.86 |
8 C |
615 |
9.94 |
0.035 |
Table 4: Field measurements of wetland piezometers selected by Dr. Stroh. Measurements were taken on 2/23/2001.
The pH values of wells were generally slightly acidic, which is expected in natural waters containing ions. The alkalinity for most wells is in the range of 40-50 ppm, with the exception of an alkalinity of 12.43 for piezometer 2 of Houston Creek (Table 3). Our conductivity data from B lot and the wetlands may be off because the samples were not analyzed at the same temperature as the conductivity standards, and conductance varies with temperature. Our dissolved oxygen concentrations are slightly higher than groundwater in equilibrium with air, which would have a DO content of 8.2 mg/L. This indicates that oxygen is available for aerobic reduction in the wetlands.
|
Piezometer |
Cl |
NO2 |
NO3 |
PO4 |
SO4 |
|
3 |
4.05 |
0.00 |
0.00 |
0.00 |
6.13 |
|
Manhole |
2.92 |
0.00 |
1.69 |
0.00 |
2.77 |
|
2b |
1.54 |
0.00 |
0.257 |
0.00 |
0.594 |
|
2a |
7.19 |
0.00 |
0.00 |
0.00 |
2.28 |
Table 5: Anion concentrations of Selected Wetland Piezometers. Water samples were collected and analyzed on 2/23/2001. All concentrations are in mg/L.
|
Piezometer |
Cl |
NO2 |
NO3 |
PO4 |
SO4 |
|
P1 |
1.71 |
0.00 |
0.695 |
0.00 |
1.72 |
|
P2 |
1.42 |
0.00 |
0.842 |
0.00 |
0.897 |
|
P3 |
2.97 |
0.617 |
0.173 |
0.885 |
1.81 |
|
P4 |
1.47 |
0.00 |
0.571 |
0.00 |
2.27 |
Table 6: Anion concentrations of Houston Creek (B-lot) piezometers. Water samples were collected and analyzed on 1/20/2001. All concentrations are in mg/L.
|
Piezometer |
Cl |
NO2 |
NO3 |
PO4 |
SO4 |
|
P1 |
1.03 |
0.00 |
0.885 |
0.00 |
0.238 |
|
P2 |
0.802 |
0.00 |
0.939 |
0.00 |
0.218 |
|
P3 |
1.09 |
0.00 |
0.464 |
0.00 |
0.367 |
|
P4 |
0.812 |
4.96 |
0.624 |
0.00 |
0.491 |
Table 7: Anion concentrations of Houston Creek (B lot) piezometers. Water samples were collected and analyzed on 3/13/01. All concentrations are in mg/L.
Only one sample had noticeable phosphate concentrations. No nitrate was present except for well P4 on March 13th. The anion data for the different piezometers in B lot is otherwise completely comparable. However, the wetlands piezometers are not. Piezometer 2b stands out the most, as its chloride, sulfate and pH values were much lower than those of the other wetland samples.
Discussion
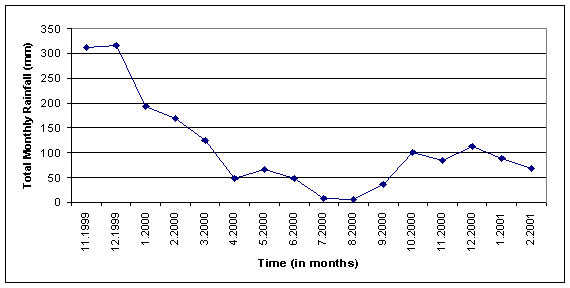
Piezometer
Elevations and Rainfall
Figure 4: Monthly rainfall totals (mm) for TESC, from TESC weather center.
Figure 4 depicts the monthly rainfall on the Evergreen Campus from 11/1999 to 02/2001. The general trend shows a dramatic decrease in precipitation from the winter of 1999 to the winter of 2000. Rainfall decreased at least 200 mm from 1999 to 2000 in the month of December. The correlation between decline in rainfall totals and decline in monitoring well water elevations was expected. With significantly less (62% on average, Table 1) precipitation falling on the TESC campus, the aquifer cannot recharge at the same rate. The difference between the relative change of rainfall totals and the relative change in monitoring well water elevations is interesting. The large difference indicates that the aquifer being monitored may not recharge by direct infiltration of rainfall on campus alone. Other possible sources that may contribute to recharge are equilibrium relationships with losing streams and lakes or infiltration and recharge from off-campus sources.
Both rainfall and piezometer static water height decreased with time (see Figures. In an attempt to find a correlation between rainfall and static H20 elevation, the rainfall totals and average static H20 elevations for 4 months from 1999-2000 were compared to the rainfall and average static elevation for the Motor Pool piezometers in the same 4 months of 2000-2001 (Table 1).
The correlation between decline in rainfall totals and decline in monitoring well water elevations was expected. With significantly less (62% on average, Table 1) precipitation falling on the TESC campus, the aquifer cannot recharge at the same rate. The difference between the relative change of rainfall totals and the relative change in monitoring well water elevations is interesting. The large difference indicates that the aquifer being monitored may not recharge by direct infiltration of rainfall on campus alone. Other possible sources that may contribute to recharge are equilibrium relationships with losing streams and lakes or infiltration and recharge from off-campus sources.
Wetlands
The water chemistry data from wetlands piezometers 2a and 2b (two aquifers that are quite close to each other) supports the presence of two distinct aquifers, since the conductivity, pH, temperature, and chloride concentrations are significantly different. The screen depths of these piezometers are not currently known. To determine the familiar characteristics of each aquifer, water could be analyzed from a nested piezometer with known depths. Our measured DO values were greater than the oxygen content for standing water in equilibrium with the atmosphere. This leads us to conclude that wetland soils are oxic, and the high concentration of dissolved oxygen makes this an interesting site for field study of redox balances.
B Lot
Our two analyses of B lot piezometers show a dip in chloride and sulfate levels over one month’s time(see tables 4 and 5). This may indicate that the groundwater on the second sample may have had a shorter residence time in the soil. Rainfall data (from the Evergreen Weather Server) shows that it rained 4mm on the day the second sample was collected, while it has not rained only 1 mm before and during. In his study of Houston Creek and the B lot piezometers Spike Minczeski theorized that the creek and the aquifers monitored with the piezometers were related. The creek loses to the aquifer during rainy times and gains from it during dry times. So changes in aquifer chemistry could indicate whether the creek is losing to or being recharged by the creek. Our current results are inconclusive due to the low concentrations (comparable to rainwater) of anions present.
The March sampling of B lot also showed an increase in the nitrate levels of Piezometer 4. (table 5). A budding alder was located near this piezometer, while the alders near other piezometers appeared dormant. So this jump was probably due to early spring nitrogen fixing by the budding alder.
Conclusions
While there is decent amount of data concerning the
surface and groundwater flow on the TESC campus, the data on water chemistry
and soils is quite limited in these areas.
This makes it very hard to come up with conclusions about the
interactions of aquifers in this region.
Future directions for research are discussed below.
Piezometer Mapping
Throughout this project we have been plagued by an abundance of data and a scarcity in relatable records. Specifically, we have had problems dealing with inconsistent piezometer labeling, incomplete data records and a myriad of coordinate systems. To adequately address this scope of data, we recommend a consolidation of data to re-establish a baseline for future study:
- All GIS data translated to State Plane Nad 27 South, in feet.
- Create a baseline Arc View Project.
- Review all piezometers for ongoing study value. Ensure all are capped.
- Decommission all unusable piezometers.
- Designate a detailed numbering system. And stencil all piezometers permanently.
- Survey all viable piezometers; confirm depth, elevation, and location.
The scope or purpose of this baseline should reflect the goal of providing a single point database for future investigations of The Evergreen State College groundwater.
Soil data:
The study of the wetland area (Smith and Callison 2000) contains a fairly complete soil description for that area although no Munsell color data is included. The B-C Hydrogelogy paper (Minczesky 2000) also contains soil data for four of the piezometers in the B lot area. Other areas need to have soil data so that further conclusions can be drawn about the chemical and physical nature of the underlying aquifers.
Water Chemistry
Several water chemistry analyses have been done on the piezometers in the B lot area and the wetlands. There is not enough data at this point to draw any conclusions. Additional sampling and testing needs to be done on these sites in order to determine baseline concentrations of chemical constituents. Continued analyses of springtime nitrogen ion concentrations in B lot should prove fruitful, as the area has an abundance of nitrogen-fixing alder trees.
Forest
types
There is currently a good deal on information on Forest types that was not available for this paper. This information could supplement studies of biogeochemical cycles in shallow groundwater piezometers.
Our linked GIS map should assist future hydrological studies at Evergreen by enabling them to quickly reference the results of earlier research.
References
Benz, 1995, in Jenkins. Remediation Excavation Report- Preliminary I.R.A.P. Report, The Evergreen State College, Motor Pool and Facility Shops Site. Fred Benz, P.E., October 25, 1995
Callison, E. and Smith, D. 2000. Groundwater Study on Projected Wetland on the Evergreen State College. Eli Callison and Dan Smith, Wetlands 2000, The Evergreen State College.
Jenkins, P. G., 1999. The Evergreen State College Motor Pool Leaking Underground Storage Tank Site Groundwater Characterization Study. Pamela G. Jenkins, P.E., Creative Environmental Technologies, Inc. December 17, 1999
Minczeski, S. B-C Creek Hydrogeology.
Noble, J. B and Wallace Geology and Ground-Water Resource of Thurton County, Wahington, Volume 2 (1966)
Appendix 1:Instructions for measuring water level elevation in monitoring wells.
J. Stroh
April 200
Looks like the levels will be in decline. I suggest measuring every two weeks, but at least monthly as required. Last measurement April 11 2000.
Equipment needed:
1. Key for the locks
2. Solinst water depth meter
3. Ratchet wrench with 9/16 socket
4. Latex gloves
5. Paper towels
6. Two buckets
7. Wash rag
8. Clean towel
9. Write in the rain notebook and 3H pencil
Most of this is in the Solinst box in the geology lab, Lab II room 3239.
Before going to the site clean 25 feet of the Solinst meter with warm soapy water (Alconox or other lab soap). Rinse and towel dry.
Make sure battery is OK with check switch.
At the site remove the steel covers in the order 1 through 4 (N, E, S, W). The well number is on the cover casing. Use the paper towels to keep from getting rust on your hands. Well #4 might need bailing (rain water collects in it). I use a paper cup.
Then remove the lock and open the cap, in the same order. Let the well stabilize as pressure or vacuum might build up with changes in water lever (the caps are air tight). By the time you have uncapped all the wells and returned to number one ample time for equilibration will have passed.
Put on the latex gloves. Turn on the meter and measure the depth to water indicated by the buzzer and the light on the meter. Measure the depth to the nearest 0.01 foot at the black mark or the highest point on the PVC pipe. Record. Do this in the order 1 2 3 4.
Clean the tape between each well. An easy way is to use one rag with soapy water followed by a rinse rag or towel as you retract the tape on to the spool.
Replace the caps, gently but firmly, and lock. Replace the steel covers. I do this after measuring all the wells to keep cleaner.
Record these data in the Excel spreadsheet. (On Masu Groups 2 Hydro-00 Spikes data Static Elevations.xls).
|
Appendix
2 B
Lot Piezometers |
MW # 1 |
MW # 2 |
MW # 3 |
MW # 4 |
|
Elevation of top
of peizometer (ft) |
164.67 |
161.23 |
162.85 |
161.47 |
|
Depth of peizometer
(ft.) |
13.81 |
8.57 |
8.08 |
5.51 |
|
Date |
Elevation (ft.) |
Elevation (ft.) |
Elevation (ft.) |
Elevation (ft.) |
|
2/1/2000 |
154.87 |
159.11 |
157.50 |
159.71 |
|
2/9/2000 |
154.32 |
159.08 |
157.17 |
159.57 |
|
2/15/2000 |
154.38 |
158.89 |
157.55 |
159.42 |
|
2/26/2000 |
154.17 |
158.53 |
156.05 |
159.07 |
|
4/11/2000 |
153.26 |
158.83 |
154.9 |
159.1 |
|
4/21/2000 |
152.57 |
158.53 |
154.62 |
158.91 |
|
5/1/2000 |
153.15 |
157.33 |
155.24 |
159.37 |
|
5/15/2000 |
146.42 |
143.73 |
147.3 |
150.07 |
|
1/20/2001 |
151.57 |
154.53 |
158.8 |
157.77 |
|
2//3/2001 |
151.58 |
156.06 |
159.32 |
157.89 |
|
3/13/2001 |
150.86 |
155.85 |
159.02 |
158.02 |
Appendix 3: Motor Pool Monitoring Well Elevations
|
|
Static elev. |
Static elev. |
Static elev. |
Static elev. |
|
|
|
Date |
MW # 1 |
MW # 2 |
MW # 3 |
MW # 4 |
Delt 3-1 |
Delt 2-4 |
|
top of Piezometer |
145.06 |
144.61 |
140.91 |
146.01 |
|
|
|
Nov 24 1999 |
120.27 |
123.1 |
124.96 |
122.64 |
4.69 |
0.46 |
|
Dec 10 1999 |
121.59 |
125.01 |
127.16 |
124.16 |
5.57 |
0.85 |
|
Dec 19 1999 |
124.44 |
127.94 |
130.27 |
127.36 |
5.83 |
0.58 |
|
Dec 26 1999 |
124.57 |
127.5 |
130.38 |
127.1 |
5.81 |
0.4 |
|
Jan 02 2000 |
124.21 |
126.98 |
128.7 |
126.57 |
4.49 |
0.41 |
|
Jan 09 2000 |
124.77 |
127.63 |
129.51 |
127.26 |
4.74 |
0.37 |
|
Jan 17 2000 |
125.94 |
128.75 |
130.66 |
128.4 |
4.72 |
0.35 |
|
Feb 11 2000 |
126.81 |
129.06 |
129.51 |
128.51 |
2.7 |
0.55 |
|
Mar 21 2000 |
127.54 |
129.57 |
131 |
129.29 |
3.46 |
0.28 |
|
Apr 11 2000 |
126.58 |
129.35 |
129.5 |
128.06 |
2.92 |
1.29 |
|
Apr 21 2000 |
125.82 |
127.6 |
128.86 |
127.3 |
3.04 |
0.3 |
|
May 06 2000 |
125.69 |
127.2 |
128.41 |
126.9 |
2.72 |
0.3 |
|
May 20 2000 |
125.15 |
126.76 |
127.81 |
131.25 |
2.66 |
-4.49 |
|
June 05 2000 |
124.16 |
126.16 |
127.42 |
125.87 |
3.26 |
0.29 |
|
June 23 2000 |
123.71 |
125.89 |
127.28 |
125.65 |
3.57 |
0.24 |
|
July 20 2000 |
122.42 |
124.56 |
125.95 |
124.26 |
3.53 |
0.3 |
|
Aug 21 2000 |
120.81 |
122.9 |
124.29 |
122.6 |
3.48 |
0.3 |
|
Oct 19 2000 |
118.44 |
120.71 |
122.2 |
120.43 |
3.76 |
0.28 |
|
Nov 10 2000 |
118.1 |
120.51 |
122.09 |
120.2 |
3.99 |
0.31 |
|
Nov 17 2000 |
117.75 |
120.22 |
121.83 |
119.9 |
4.08 |
0.32 |
|
Dec 18 2000 |
117.36 |
120.11 |
121.82 |
119.76 |
4.46 |
0.35 |
|
Jan 25 2001 |
117.61 |
120.92 |
122.86 |
120.48 |
5.25 |
0.44 |
|
Feb 23 2001 |
118.01 |
121.62 |
123.75 |
121.16 |
5.74 |
0.46 |
|
Mar 13 2001 |
117.83 |
120.96 |
122.95 |
120.67 |
5.12 |
0.29 |
Static Elevation is the actual elevation of the water compared to sea level.
October 17, 2000
EPA method 9056A describes the use of ion
chromatography for analysis of solid waste.
In this method they describe how to prepare a high, intermediate, and
low range standard. The high range
standard is prepared from 1000 mg/L stock solutions. The intermediate and low standards are prepared by diluting the
high and intermediate standards respectively.
For the high range standard the method recommends the following
concentrations in mg/L: chloride 10, nitrite 20, nitrate 30, phosphate 50, and
sulfate 100. Intermediate standard is
made from a 1:10 dilution of high standard, and low standard is made from a 1:5
dilution of intermediate standard. This
approach is useful because it saves a great deal of pipetting. For this week, let’s modify the method as
follows:
I will prepare a single stock solution that contains:
Chloride 1000 mg/L
Nitrite - N 1000 mg/L
Nitrate -N 1000 mg/L
Phosphate –P 4000 mg/L
Sulfate 4000 mg/L
High-Range Standard
Using a 10 mL transfer pipette and a 1000 mL volumetric flask, transfer 10.00 mL of the stock solution to the volumetric flask and dilute to 1000 mL.
Intermediate-Range Standard
Using a 10 mL transfer pipette and a 100 mL volumetric flask, transfer 10.00 mL of the High-Range standard to the volumetric flask and dilute to 100.0 mL.
Low-Range Standard
Using a 20 mL transfer pipette and a 100 mL volumetric flask, transfer 20.00 mL of the Intermediate-Range standard to the volumetric flask and dilute to 100 mL.
|
|
High-Range (mg/L) |
Intermediate Range (mg/L) |
Low Range (mg/L) |
|
Chloride |
10 |
1.0 |
0.20 |
|
Nitrite – N |
10 |
1.0 |
0.20 |
|
Nitrate –N |
10 |
1.0 |
0.20 |
|
Phosphate –P |
40 |
4.0 |
0.80 |
|
Sulfate |
40 |
4.0 |
0.80 |