Go To: Chemistry
Answers Physics Answers
Chemistry: Chapter
8 - Spontaneous Processes & Thermodynamic Equilibrium
3.
a) There are 36 "microstates" for the combinations of numbers that can come up on two dice, e.g., 6n, because there are six possible values of n dice.
b) Only one of the 36 microstates will have a six on both die, thus the probability is 1/36. By the way, there are six microstates that will give a total roll of seven, thus the chances of rolling a seven is 1/6. Think about this the next time you are at a casino.4.
a) The number of microstates for the sytem described is 34, or 81.b) The probability that all four molecules are in the leftmost third at the same time is 1/81.
11.
a) The thermodynamic efficiency of the engine is:
b)
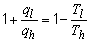
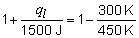
ql = -1000 J
c) In any complete cycle, the internal energy of the fluid in the engine remains constant, i.e., DE = 0. By the first law, DE = q + w = qh + ql + w. Then, 0 = 1500 + (-1000) + w. Then w = -500 J.
17. Since the expansion is isothermal, DE
= DH = 0.
The work performed is given by:
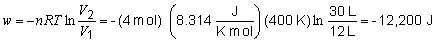
By the first law, DE = 0 = q + w; q = 12,200 J.
The change in entropy is then,
19) To find the total entropy change for this problem,
the entropy change of each step must be calculated individually.
Step 1:
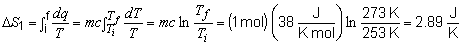
Step 2:
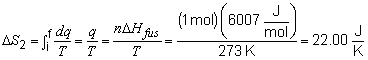
Step 3:
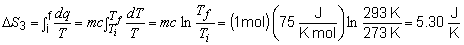
The total change in entropy is then:
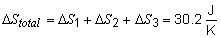
Since the process is reversible, DSuniverse = 0 and DSsurr = -30.2 J K-1.
22. At constant pressure, q = DH.
So,
The total change in entropy is positive, as it must be for any spontaneous process.
Physics: Chapter 22 -
Entropy & The Second Law of Thermodynamics
31)
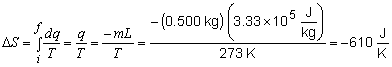
33)
![]()
36) In this problem we arbitrarily define the system as the horseshoe
(Fe) and the surroundings as the water. To find the change in entropy,
we must first calculate the temperature of the system and surroundings
after thermal equilibrium is established.
qsystem = -qsurroundings(mcDT)Fe = -(mcDT)H2O
(mcDT)Fe = -(mcDT)H2O
(mc)Fe (Tf -Ti, Fe) = -(mc)H2O(Tf -Ti, H2O)
Tf(mc)Fe - (mc)Ti, Fe = -Tf(mc)H2O + (mc)Ti, H2O
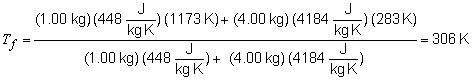
To find the total entropy change of the universe:
39)
![]()
Since the volume doubles, Vf = 2 Vi. Then,
![]()
The temperature of the gas does not change; since it was
expanding into a vacuum, no work was done so there is no change in the
internal energy.
41) It is useful to treat these as independent samples of expanding gases. There will be an increase in entropy as the hydrogen expands, and an increase in entropy as the oxygen expands. The total entropy change will be the sum.
![]()
![]()
![]()
61) In this problem, arbitrarily define the cream as the system and the coffee as the surroundings. As in problem 36, to find the total entropy change, you must first find the equilibrium temperature. Using the formula derived in #36, we have
![]()
But since we are assuming that the heat capacities of
the coffee and cream are the same, they cancel out of the equation.
![]()
![]()
![]()
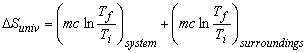
![]()
![]()
66) The maximum possible efficiency of any heat engine
is that provided by a Carnot engine. Given the temperature of the high
temperature reservoir and the low temperature reservoir, the Carnot efficiency
is:
Since the device in question operates at an efficiency
of 0.61 (61%) it is clearly in violation of the second law of thermodynamics
- it is a fraud.