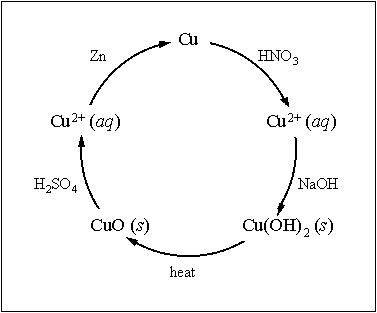
Figure 1. The cycle of copper reactions to be performed in this experiment
Conservation of Mass: A Cycle of Copper Reactions
Purpose. The goal of this experiment is to introduce you
to several classes of chemical reactions: oxidation/reduction, precipitation,
decomposition and acid/base neutralization. The reactions will be performed
in a specific order and, when completed, will regenerate the starting material,
pure copper. Since you will not add copper to the reaction at any point
after the initial step, it should be possible to recover all of the copper
at the end of the cycle, thereby illustrating the Law of Conservation of
Mass. You are asked to practice your observational skills by recording
the changes that occur at each step of the cycle. Color changes, the generation
of gases or precipitates and changes in temperature are all examples of
the types of phenomena you should be looking for when studying chemical
reactions.
Introduction. Thousands of individual chemical reactions
can be classified into a very small number of reaction types. In this experiment,
several different types of reactions will be performed to transform copper
into a variety of its compounds. It is important to realize that each product
contains copper and that the total number of copper atoms involved in each
step is the same. Therefore, at the end of the cycle, the mass of copper
recovered should equal the mass that was originally used. You will calculate
the percent yield of copper at the end of the cycle to determine the "efficiency"
of the process.

Figure 1. The cycle of copper reactions to be performed in this experiment
The cycle of reactions to be performed is shown in Figure 1. Beginning with pure copper at the top of the figure, these are:
Reaction 1: Oxidation of metallic copper with nitric acid (HNO3). The balanced equation is:
8 HNO3 (aq) + 3 Cu (s) + O2 (g) ® 3 Cu(NO3)2 (aq) + 4 H2O (l) + 2 NO2 (g)
Reaction 3. Thermal decomposition of copper (II) hydroxide to copper (II) oxide. Compounds that are stable under standard conditions often become unstable at elevated temperatures. Many times this can result in the loss of gases, for example, the loss of carbon dioxide from calcium carbonate:
CaCO3 (s) ® CaO (s) + CO2 (g)
When copper (II) hydroxide is heated to roughly 100° C, it decomposes to copper (II) oxide according to the following reaction.
Cu(OH)2 (s) ® CuO (s) + H2O (l)
Reaction 4. Reaction of copper (II) oxide with sulfuric acid.
CuO (s) + H2SO4 (aq) ® CuSO4 (aq) + H2O (l)
Reaction 5. Reduction of copper (II) with zinc.
Cu2+ (aq) + Zn (s) ® Cu (s) + Zn2+ (aq)
After the reaction is complete, the excess zinc must be removed from
the copper/zinc mixture in order to purify the copper and accurately measure
its mass. To accomplish this, the mixture is treated with hydrochloric
acid. At this point the solid, pure copper can be isolated, dried and weighed.
Experimental Procedure.
Safety Notes.
· Wear Safety Glasses at ALL TIMES. You will be using nitric, sulfuric and hydrochloric acids as well as sodium hydroxide– all are damaging to skin, clothing and especially your eyes.Reaction 1. Get a piece of copper foil and cut an approximately 0.5 g sample. If it is not bright and shiny, clean it with a piece of steel wool, rinse with water and dry with a paper towel. Then get an accurate mass measurement using an analytical balance. Place in a 250 mL beaker and add about 4 mL of concentrated nitric acid slowly and carefully. Record in your notebook a description of what you see. After the copper has dissolved, add 10 mL of deionized water to dilute the sample for step 2.· Perform Reaction 1 in a fume hood. Toxic NO2 is produced.
· Hydrogen gas is evolved in Reaction 5. Keep your apparatus away from open flames.
Reaction 2. While stirring with a glass stirring rod, add approximately
15 mL of 6 M NaOH. Record your observations in your notebook. Dilute the
solution with deionized water to about 100 mL in preparation for step 3.
Reaction 3. Add a magnetic stir bar and place on a heatable stir
plate. Boil gently while stirring for about 4 minutes. Record any
changes that occur in your notebook. Remove the beaker from the heat and
allow to cool. Add 40 mL of deionized water into a second clean beaker
and begin heating.
Prepare a filter paper and funnel to filter the copper (II) oxide. Use
a 250 mL beaker to collect the filtrate, supporting the funnel with a funnel
support or iron ring on a ringstand. Filter the copper (II) oxide. The
filtrate should be colorless and free of any solids. Transfer the last
traces of solid material from the beaker using a stream of deionized water
from a wash bottle. Use the deionized water that you have been heating
to wash the solid collected in the filter paper. Pour about 5 mL of the
hot water through the filter. Repeat three times. Leave the filtration
apparatus in place for the next step. In your notebook, describe the appearance
of the collected solid.
Reaction 4. Dissolve the CuO by carefully pouring about 15 mL
of 3 M sulfuric acid directly through the residue on the filter into a
250 mL beaker. Record any changes that you see. If the solid is not completely
dissolved the first time, replace the collection beaker with a clean new
one and pour the acid in the first beaker through the filter again. Pour
very carefully so as not to lose any of the liquid. Repeat this procedure
as often as necessary to dissolve all of the solid. It may take four or
five times.
Once the solid is dissolved, you need to collect all of the copper (II)
containing solutions in the same beaker. Rinse down the walls of the collection
beaker that is not in position below the filter with deionized water from
a wash bottle and pour the rinse water through the filter into the other
beaker. Wash the empty filter paper with three or four 5 mL portions of
cold deionized water and collect the washings in the beaker containing
the acid solution.
Reaction 5. Add about 2 g of zinc metal to the copper (II) solution
and stir rapidly. Hydrogen gas bubbles should appear – keep flames away!
Record your observations in your notebook. The reaction between the zinc
and copper (II) ion will be complete when the blue color of the copper
solution is gone. If any blue color remains after the zinc has been consumed,
add approximately 0.5g of additional zinc. Record this in your notebook.
When the copper has been completely reduced, decant most of the solution,
even if the zinc is still generating gas. Add 25 mL of 3 M HCl to speed
up the rate of zinc oxidation. When no more bubbles are seen, proceed to
the epilogue. Record your observations.
Epilogue. Allow the copper metal to settle to the bottom of the
beaker. Carefully decant the supernatant. Do not try to pour off all of
the liquid – it is better to leave a small amount of liquid than to lose
any precipitate. Wash the copper metal precipitate with three 50 mL portions
of water, removing each portion by decantation. Weigh a clean, dry evaporating
dish and record the mass. Transfer all of the copper precipitate into the
evaporating dish (use a rubber policeman – in good condition! – and a wash
bottle to help with this). Let the solid settle in the evaporating dish
and carefully decant most of the liquid. Set the evaporating dish in a
110° C oven for 30 minutes to evaporate
the water. Remove from the oven and allow to cool to room temperature (place
on a paper towel with your name on it). Weigh the evaporating dish and
calculate the mass of copper recovered and your percent yield using the
following equation:
![]()
Report Sheet
Name: ______________________
Observations. Describe what happens physically in each of the
following reactions. Be specific with regard to color changes, gases formed,
etc.
Reaction 1. Oxidation of copper with nitric acid.
Reaction 2. Precipitation of copper (II) hydroxide with sodium hydroxide.
Reaction 3. Decomposition of copper (II) hydroxide to copper (II) oxide.
Reaction 4. Reaction of copper (II) oxide with sulfuric acid.
Reaction 5.
b) Oxidation of excess zinc with hydrochloric acid.
Experimental Data:
Mass of copper foil ______________
Mass of copper recovered ______________
Percent Recovery ______________