![]()
Ruthenium will not float since it has
a density greater than that of water.
b)
c)
Review Assignment #1
Chapter 1
4.
a) air: homogeneous mixture
b) tomato juice: heterogeneous mixture
c) iodine crystals: pure substance (it is an element)
d) a nickel: homogeneous mixture
(nickels are composed of an alloy that is 75% copper and 25% nickel; metallic
alloys are homogeneous mixtures)
14. Chemical & Physical Properties of Zinc
Physical properties:· silver grey metalChemical properties:
· melting point = 420o C
· hardness = 2.5
· density = 7.13 g/cm3· reacts with dilute sulfuric acid to yield hydrogen and dissolves
· reacts with oxygen to give zinc oxide
24.
a)26.
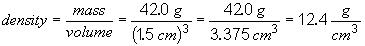
Ruthenium will not float since it has a density greater than that of water.
b)
c)
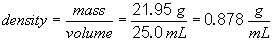
The measured density is consistent with the tabulated value
b)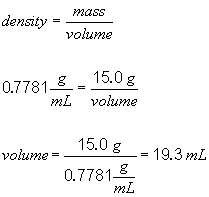
c) The volume of the sphere is:

Thus, the mass of the sphere is:
32.
a) 1.689 ´ 10-3 km (4 significant figures)b) 0.0234 m2 (3 significant figures)
c) 7,194,300 cm (5 significant figures)
d) 435.983 K (6 significan figures)
e) 204.080 g (6 significant figures)
36.
a) 1.44 ´ 105b) 9.75 ´ 10-2
c) 8.90 ´ 105
d) 6.76 ´ 104
e) 3.40 ´ 104
f) -6.56 ´ 100
38.
|
a)
|
|
|
b)
|
|
|
c)
|
|
|
d)
|
|
44.
|
a)
|
|
|
b)
|
|
|
c)
|
|
|
d)
|
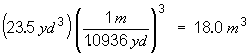
|
|
e)
|
|
|
f)
|
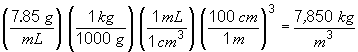
|
49. To answer this problem, you have to realize that the volume of the ingot is the same as the volume of the drawn wire. You can get the volume of the ingot using the mass and density information provided in the problem. The volume of the wire is given by the formula for the volume of a cylinder, V = pr2 h, where h is the height of the cylinder, or in this case the length of the wire. So,
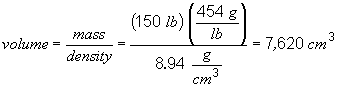
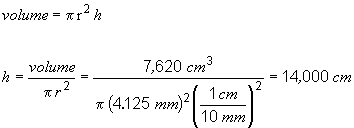
To convert this to feet,
69. This problem can
be solved with a creative application of units
a)
b)
c)
d)
Chapter 2
14.
| nuclide |
|
|
|
|
a)
|
phosphorus-32 |
|
|
|
b)
|
chromium-51 |
|
|
|
c)
|
cobalt-60 |
|
|
|
d)
|
technetium-99 |
|
|
|
e)
|
iodine-131 |
|
|
|
f)
|
thallium-201 |
|
|
16.
| Symbol |
|
|
|
|
|
| Protons |
|
|
|
|
|
| Neutrons |
|
|
|
|
|
| Electrons |
|
|
|
|
|
| Atomic No. |
|
|
|
|
|
| Mass No. |
|
|
|
|
|
22.
carbon: non-metalsilicon: metalloid
germanium: metalloid
tin: metal
lead: metal