b)
![]()
c)
![]()
Review
Assignment #4
Chapter 4: Solution Concentration & Stoichiometry
52.
a)57.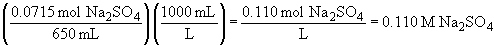
b)
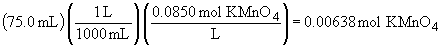
c)
a)69.
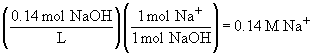
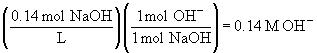
b)
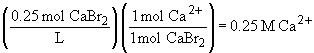
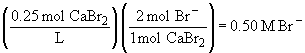
c) 0.25 M CH3OH (this compound is not ionic)
d) To answer this question, first find the total number of moles of each species and then divide by the new volume (75.0 mL)
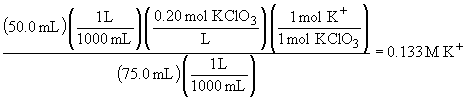
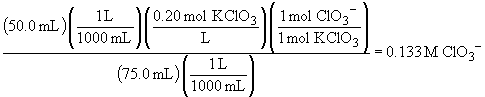
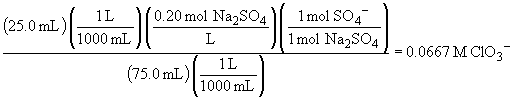
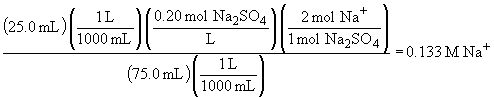
70.
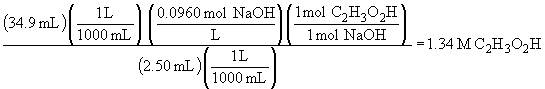
Converting the above concentration to grams per quart gives:
90.
Chapter 13: Colligative Properties
28.
a)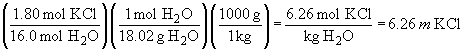
b)
30. a) mass percentage:
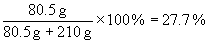
b) mole fraction:
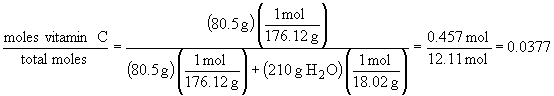
c) molality
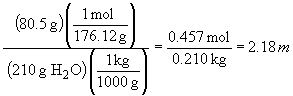
d) molarity. To calculate molarity, the volume of the solution needs to be calculated; since the total mass (290.5 g) and density (1.22 g/mL) are known, we can proceed as follows:
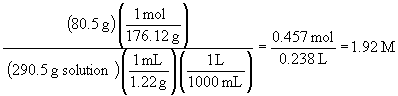
33. a)
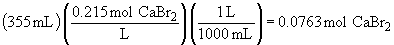
b) There is no simple set-up for this problem. It issolved by multiplying the sample mass by the mass fraction of KCl in the solution; this gives the mass of KCl. Then convert to moles as usual.
mass fraction:
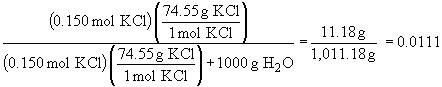
mass KCl:
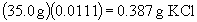
moles KCl:
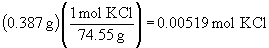
Note that the book answer of 0.00525 mol is not correct.
c)
40.
a) mass percent: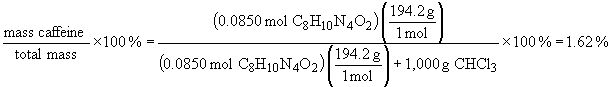
b) mole fraction:
44. The vapor pressure over a solution is given by:
Psolution = csolvent P°solvent
In this question, the mole fraction of the two solutions is not the same as shown below. Since the mole fractions are not the same, neither will be the vapor pressures. The sucrose solution will have a very slightly higher vapor pressure.mole fraction glucose solution:
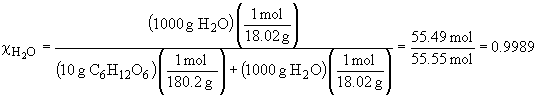
46. a) To find the vapor pressures of this solution,
first calculate the mole fraction of the solvent:
50. a) 0.50 m glucose in ethanol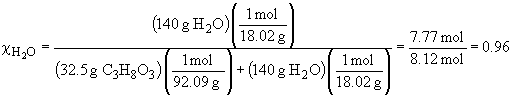
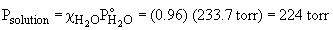
b) To find the mass necessary to reduce the vapor pressure to a given level, first solve for c.
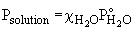
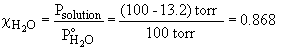
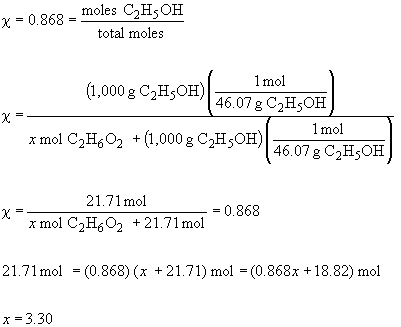
The mass is then,
boiling point elevation:
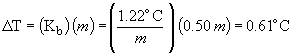
boiling point = 78.4° C + 0.6 ° C = 79.0 ° C
freezing point depression:
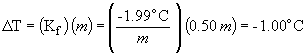
freezing point = -114.6 ° C - 1.0 ° C = -115.6 ° C
b) first, calculating the solution molality:
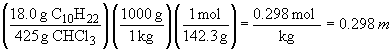
boiling point elevation:
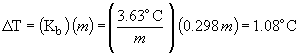
boiling point = 61.2° C + 1.1 ° C = 62.3 ° C
freezing point depression:
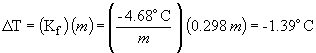
freezing point = -63.5 ° C - 1.4 ° C = -64.9 ° C
c) the solution molality is:
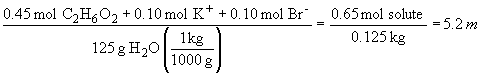
boiling point elevation:
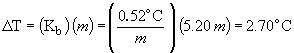
boiling point = 100.0° C + 2.7 ° C = 102.7 ° C
freezing point depression:
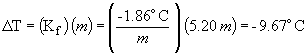
freezing point = 0.0 ° C - 9.7 ° C = -9.7 ° C
52. The order of freezing points will be: 0.075 M
LiBr < 0.030 M Zn(NO3)2 < 0.075 M glucose.
This is because the freezing point depression depends on the total number
of solute particles, i.e., ions and/or molecules.
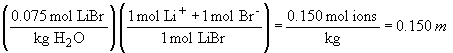
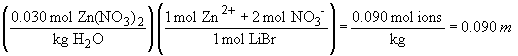
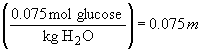
Therefore, the LiBr solution will show the greatest freezing point depression, followed by the Zn(NO3)2 and glucose in that order.
53.
55. In this question, the molality of the solution
can be determined from the freezing point depression; the mass information
can then be used to determine the molecular weight.
DT = 4.1° C - 5.5° C = -1.4° C
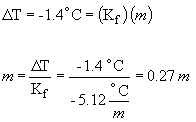
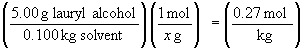
x = 185 g/mol
Chapter 5: Introduction to Thermochemistry
3.
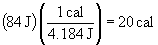
When the golf ball hits a tree, the kinetic energy is converted into sound (kinetic energy of air molecules), heat, and work (the damage the ball does to itself or the tree on impact).
28 a) The reaction is endothermic (DH
is positive)
b)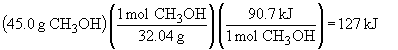
c)
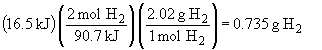
d) For the reverse reaction, we can write:
CO (g) + 2 H2 (g) ® CH3OH (g) DH = -90.7 kJ
30a)
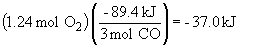
b)
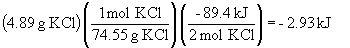
c) For the reverse reaction,
2 KCl (s) + 3 O2 (g) ® 2 KClO3 (s) DH = +89.4 kJ
37. a) 4.184 J/(g °
C)
b)
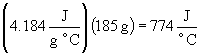
c)
39.
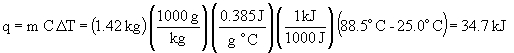
44.
![]()
Heats of combustion:
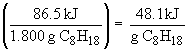
53. Given the following reactions:
H2 (g) + F2 (g) ® 2 HF (g) DH1 = -537 kJC (s) + 2 F2 (g) ® CF4 (g) DH2 = -680 kJ
2 C (s) + 2 H2 (g) ® C2H4 (g) DH3 = +52.3 kJ
The following can be written:
C2H4 (g) ® 2 C (s) + 2 H2 (g) DH = - DH3 = -52.3 kJ
2 C (s) + 4 F2 (g) ® 2 CF4 (g) DH = 2 DH2 = -1,360 kJ
2 H2 (g) + 2 F2 (g) ® 4 HF (g) DH = 2 DH1 = -1,074 kJ
______________________________ ____________________C2H4 (g) + 6 F2 (g) ® 2 CF4 (g) + 4 HF (g) DH = -2,486 kJ
58.
a) ½ H2 (g) + ½ Cl2 (g) ® HCl (g) DH = -92.3 kJb) Ag (s) + 1/2 N2 (g) + 3/2 O2 (g) ® AgNO3 (s) DH = -124.4 kJ
c) 2 Al (s) + 3/2 O2 (g) ® Al2O3 (s) DH = -1669.8 kJ
d) C (s) + 2 H2 (g) + ½ O2 (g) ® CH3OH (l) DH = -238.6 kJ
61a) 2 SO2 (g) + O2 (g)
®
2 SO3 (g)
DH° = [2 DH°f (SO3)] - [2 DH°f (SO2) + DH°f (O2)]66. In this problem, you are asked to determine the standard enthalpy of formation of B2H6. The reaction is:
DH° = [2 (-395.2 kJ)] - [2 (-269.9 kJ) + (0 kJ)] = -250.6 kJ
b) Mg(OH)2 (s) ® MgO (s) + H2O (l)
DH° = [DH°f (MgO) + DH°f (H2O, l)] - [DH°f (Mg(OH)2)]
DH° = [(-601.8 kJ) + (-285.8 kJ)] - [(-924.7 kJ)] = +37.1 kJ
c) 4 FeO (s) + O2 (g) ® 2 Fe2O3 (s)
DH° = [2 DH°f (Fe2O3)] - [4 DH°f (FeO) + DH°f (O2)]
DH° = [2 (-822.2 kJ)] - [4 (-271.9 kJ) + (0 kJ)] = -556.8 kJ
d) SiCl4 (l) + 2 H2O (l) ® SiO2 (s) + 4 HCl (g)
DH° = [DH°f (SiO2) + 4 DH°f (HCl, g)] - [DH°f (SiCl4) + 2 DH°f (H2O, l)]
DH° = [(-910.9 kJ) + 4(-92.3 kJ)] - [(-640.1 kJ) + 2(-285.8 kJ)] = -68.4 kJ
2 B (s) + 3 H2 (g) ® B2H6 (s) DH = ?The reactions given are:
| 4 B (s) + 3 O2 (g) ® 2 B2O3 (s) | DH1 = -2509.1 kJ |
| 2 H2 (g) + O2 (g) ® 2 H2O (l) | DH2 = -571.7 kJ |
| B2H6 (g) + 3 O2 (g) ® B2O3 (s) + 3 H2O (l) | DH3 = -2147.5 kJ |
These can be written as:
| 2 B (s) + 3/2 O2 (g) ® B2O3 (s) | DH = 1/2 DH1 = -1,254.6 kJ |
| 3 H2 (g) + 3/2 O2 (g) ® 3 H2O (l) | DH = 3/2 DH2 = -857.6 kJ |
| B2O3 (s) + 3 H2O (l) ® B2H6 (g) + 3 O2 (g) | DH = -DH3 = +2147.5 kJ |
| ____________________________________ | _____________________ |
| 2 B (s) + 3 H2 (g) ® B2H6 (s) | DH = +35.3 kJ |
Chapter 11: Energetics of Phase Changes
31. For this problem, we can use the relationship qsystem = -qsurroundings, where the system is the water being cooled and the surroundings is the water being evaporated. So,
32. In this problem, the system will be the water
that eventually freezes and the surroundings will be defined as the evaporating
CFC.
qsystem = -qsurroundings
33. The total heat required to convert solid ethanol
to ethanol vapor includes:
the heat required to warm the solid to the melting point, q1
the heat required to melt the solid, q2
the heat required to warm the liquid to the boiling point, q3
the heat required to vaporize the liquid, q4So, qtotal = q1 + q2 + q3 + q4
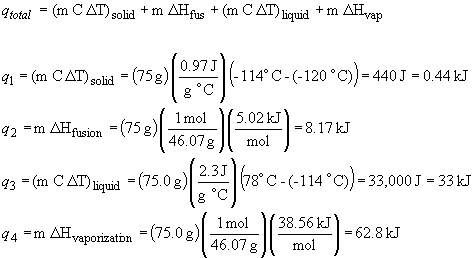
qtotal = 0.44 kJ + 8.17 kJ + 33 kJ + 62.8 kJ = 104 kJ