|
|
|
|
 |
|
 |
Review Assignment #8
Chapter 9: Bonding Theories
28.
|
|
|
|
 |
|
 |
34.
|
formula |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
36. (a) A double bond typically consists of one s
bond and one p bond. (b) A triple bond usually
consists of one s bond and 2 p
bonds. (c) Since rotation about the bond axis will greatly affect the overlap
of parallel p orbitals of a p bond, the molecule
becomes more rigid, that is, rotation is hindered, to preserve the maximum
orbital overlap.
38. The Lewis structures for N2 and N2H4 are shown below.
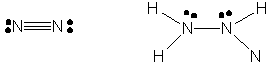
The hybridization on N2 is sp and that of N2H4
is sp3. Atoms with sp3 hybridization
typically do not participate in multiple bonding since there are no available
p orbitals to form such bonds. The only exceptions to this come with atoms
that can expand their octets, that is, elements in the 3rd row
or higher of the Periodic Table. Since nitrogen can not expand its octet,
no sp3 hybridized nitrogen (or carbon, or oxygen) can
form multiple bonds.
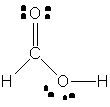
40. The CH2 carbon of ketene is sp2 hybridized;
the CO carbon is sp hybridized. (b) There are 16 valence electrons
in ketene. (c) There are four s bonds, so there
are eight valence electrons that make up these bonds. (d) There are two
p bonds, so there are four electrons that make
up those bonds. (e) There are four unpaired electrons; these are all on
the oxygen atom.
42. The bond angle will be: 1) about 120º, 2) about 120º,
and 3) about 109º. The hybridization of the central atoms of these
angles are: sp2, sp2 and sp3,
respectively.
47. (a) The s and s*
orbitals of H2+ are illustrated below. Also shown
is the energy diagram for the ion.
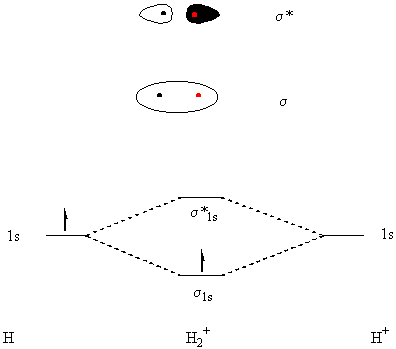
(b) There is only one electron in H2+. The Lewis dot structure is shown below - it follows none of the rules established for "normal" Lewis structures.
(c) The electron configuration for the ion is: s1s1
(d) The bond order of H2+ is ½. (Recall, the
bond order is given by the number of bonding electrons minus the number
of atibonding electrons, divided by two). (e) If the electron in the bonding
molecular orbital is promoted to the antibonding orbital, the bond order
would go from +½ to -½. This is an unstable arrangement and
the molecule would break apart to reduce the nuclear repulsion that would
exist in the absence of bonding electron interactions.
48. This problem is exactly like 47, only different. The s and s* orbitals are exactly the same, and the energy diagram is similar, but it has a total of three electrons instead of one.
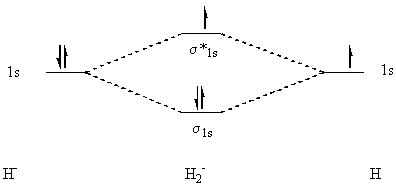
(b) A Lewis structure of H2- cannot be drawn without
exceeding the "octet" rule for hydrogen (the "duet" rule? H can only have
two electrons under normal conditions). (c) The electron configuration
for the ion is: s1s2 s*1s1
(d) The bond order of H2- is ½. (e) As explained
above, an excited H2- would have a bond order of
-½ and would be expected to decompose.
52. Using the following energy diagram, the electron configurations
and bond orders for Ne2 and Ne2+ can be
determined by filling in the orbitals according to the Aufbau Principle.
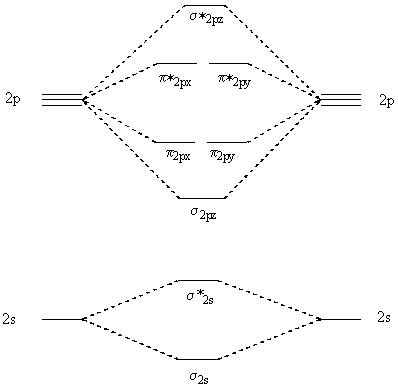
|
Species |
|
MO electron configuration |
|
|
|
|
s2s2 s*2s2 s2pz2 p2px2 p2py2 p*2px2 p*2py2 s*2pz2 |
|
|
|
|
s2s2 s*2s2 s2pz2 p2px2 p2py2 p*2px2 p*2py2 s*2pz1 |
|
Since the bond order of Ne2 is zero, it is not expected to
be stable under any conditions. Ne2+, on the other
hand, should exist under some conditions since the bonding interactions
are stronger than the anti-bonding interactions.
54. Diamagnetism is the magnetic behavior that results from a species
having no unpaired electrons. Diamagnetic materials are slightly repelled
by applied magnetic fields. Using the above energy diagram, the electron
configurations, and hence the dia- or paramagnetism of species can be predicted
|
Species |
|
MO electron configuration |
bond order |
|
|
|
s2s2 s*2s2 s2pz2 p2px2 p2py2 p*2px1 p*2py1 |
|
|
|
|
s2s2 s*2s2 s2pz2 p2px2 p2py2 p*2px2 p*2py2 |
|
|
|
|
s2s2 |
|
|
|
|
s2s2 s*2s2 s2pz2 p2px1 |
|
56. Using the energy diagram above, the electron configuration and bond
orders for N2- and N2 can be determined.
|
Species |
|
MO electron configuration |
bond order |
|
|
|
s2s2 s*2s2 s2pz2 p2px2 p2py2 |
|
|
|
|
s2s2 s*2s2 s2pz2 p2px2 p2py2 p*2px1 |
|
Since the bond order for N2- is less than that of N2, it is expected to have a weaker and shorter bond.
66.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
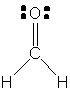 |
|
|
|
|
|
 |
|
|
Chapter 15: Introduction to Chemical Equilibria
8.
|
|
N2 (g) + O2 (g) « 2 NO (g) | ||
|
|
Ti (s) + 2 Cl2 (g) « TiCl4 (l) | ||
|
|
2 C2H4 (g)
+ 2 H2O (g) «
2 C2H6 (g) + O2 (g) |
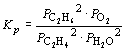 |
|
|
|
FeO (s) + H2 (g) « Fe (s) + H2O (g) | ||
|
|
4 HCl (g) + O2
(g) «
2 H2O (l) + 2 Cl2 (g) |
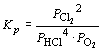 |
15. SO2 (g) + ½ O2 (g) « SO3 (g)
![]()
|
|
SO3 (g) « SO2 (g) + ½ O2 (g) | |
|
|
2 SO2 (g) + O2 (g) « 2 SO3 (g) | |
|
|
SO3 (g) « SO2 (g) + ½ O2 (g) |
19. 2 HI (g) « H2 (g) + I2 (g)
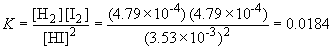
23. 2 NO (g) + 2 H2 (g) « N2 (g) + 2 H2O (g)
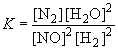
To find the equilibrium concentrations, we need to set up a table of changes, as shown below.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31. SO2Cl2 (g) « SO2 (g) + Cl2 (g)
Since the equilibrium concentration of NO is given as 0.062 M, x can be calculated as follows:0.062 = 0.100 - 2x
x = 0.019So, the equilibrium concentrations are:
[H2]eq = 0.050 - 2(0.019) = 0.012 M
[N2]eq = 0.019 M
[H2O]eq = 0.10 + 2(0.019) = 0.138 MSubstituting into the definition of K yields:
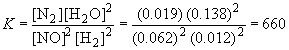
![]()
Since we are solving for [Cl2], we can rearrange the equation to:33. Br2 (g) « 2 Br (g)
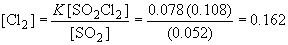
To rearrange for [Br]:
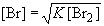
The concentration of Br2 is:
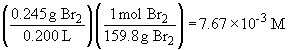
Now, [Br] can be calculated using the above equation,
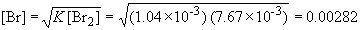
So, the mass of Br is:
b. 2 HI (g) «
H2 (g) + I2 (g)
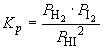
The equilibrium pressures of H2 and I2 are:
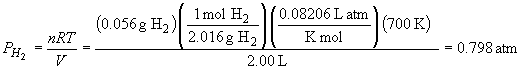
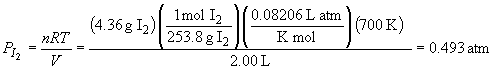
Rearranging the equilibrium expression for PHI yields:
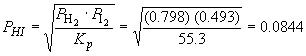
So, the mass of HI is:
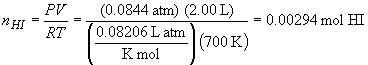
35. 2 NO (g) «
N2 (g) + O2 (g)
![]()
To find the equilibrium concentrations, we need to set up a table of concentrations as shown below:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Substituting into the equilibrium expression,
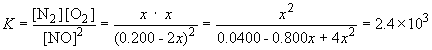
x2 = (2.4´ 103) (0.0400 - 0.800x + 4x2)
Rearranging yields,
0 = 9,599x2 -1,920x + 96
So, for the quadratic equation: a = 9,599; b = -1,920; c = 96
Solving the quadratic equation for x yields: x = 0.101 and 0.0990. Only the second solution makes physical sense as the first would give an equilibrium concentration of NO that is negative.
So, x = 0.0990
The equilibrium concentrations are then:
[NO] = 0.200 - 2(0.0990) = 0.00200 M
[O2] = 0.0990 M
[N2] = 0.0990 M
39a. PH3BCl3 (s)
«
PH3 (g) + BCl3 (g)
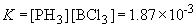
The table of concentrations that is relevant is:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Substituting into the equilibrium expression gives:
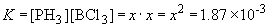
x = 0.0432
So the equilibrium concentrations are:
[PH3]eq = 0.0432 atm
[BCl3]eq = 0.0432 atm
41. I2 (g) + Br2
(g) « 2 IBr (g)
![]()
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
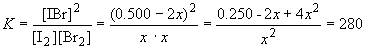
0.250 - 2x + 4x2 = 280x2
0 = 276x2 + 2x - 0.250
So, solving the quadratic equation when a = 276, b = 2 and c = -0.250, yields x = 0.0267 and -0.0339. Only the positive value of x makes physical sense. So, x = 0.0267, and the equilibrium concentrations are:
[I2] = [Br2] = 0.0267 M
[IBr] = 0.500 - 2(0.0267) = 0.447 M
44.
a. an increase in the partial pressure of a reactant will shift the reaction to the right, that is, more product will be formed.b. since the reaction is endothermic, heat can be viewed as a reactant; therefore increasing the temperature has the same effect as increasing the concentration of reactants and will shift the equilibrium to the right.
c. removing CO2, a reactant, will shift the equilibrium to the left.
d. decreasing the total pressure will have no effect on the equilibrium position since the number of gaseous molecules is the same for the reactants and products.
e. removing some of the product will shift the equilibrium to the right.
f. adding a catalyst will have no effect on the equilibrium position.
52. H2 (g) + S (s)
«
H2S (g)
The equilibrium concentrations are:
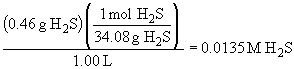
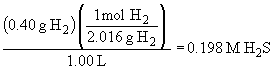
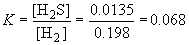
The amount of sulfur can be ignored because it is a solid material and, as such, its concentration is independent of the actual amount present.
54. A (g) «
2 B (g)
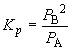
A table of pressure changes can be set up as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Since the partial pressure of A is 0.36 atm at equilibrium, we can write:62. Ni (s) + 4 CO (g) « Ni(CO)4 (g)0.36 = 0.55 - x
x = 0.19So, PB = 2x = 0.38 atm
Ptotal = PA + PB = 0.36 atm + 0.38 atm = 0.74 atm
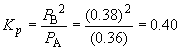
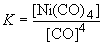
b) Since the equilibrium shifts to the left at higher temperature, the reaction as written must be exothermic.
c) By LeChâtelier's Principle, the equilibrium position shifts to the product side as products are removed from a system. In the case of the exhaust system, as the Ni(CO)4 is formed in the pipes, it is swept out with the waste gases. Thus, equilibrium with CO is never really achieved and more Ni(CO)4 is continually formed because its concentration never increases to its equilibrium value.