Caulerpa taxifolia
From marinelife1011
The Caulerpa taxifolia (Killer Algae)is a marine belonging to the class Bryopsidophyceae found in the nearshore intertidal zone along the Pacific coast of North America. Their known range is from the
| Caulerpa taxifolia | |
|---|---|
 |
|
| Scientific classification | |
| Kingdom: | Plantae |
| Phylum: | Chlorophyta |
| Class: | Bryopsidophyceae |
| Order: | Bryopsidales |
| Family: | Caulerpaceae |
| Genus: | Caulerpa |
| Subgenus: | <Phenotype> |
| Species: | C. taxifolia |
Contents |
Phylogeny
A native tropical variant of Caulerpa taxifolia exists among the tropical climate zones with local populations found in the Atlantic Ocean (West Indies and African coast), the Indian Ocean (Pakistan, Sri Lanka, and north western Australia), and the Pacific Ocean (Philippines, Indonesia, Japan, New Caledonia, and north eastern Australia) where it proliferates in limitation without issue. This marine, green alga, was first described by M.Vahl in 1802 as Fucus taxifolius and was regrouped in 1817 by C.Agardh.
Biology
Phenotype
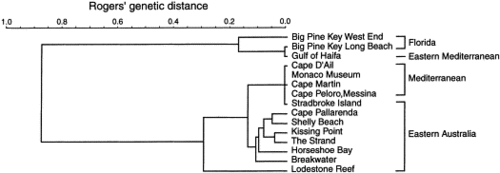
Genetic relationships of 15 Caulerpa populations using the six loci for which consistent interpretations were reliably obtained for both taxa. The metric was Rogers’ genetic distance (Rogers, J.S., 1972. Measures of genetic similarity and genetic distance. Studies in Genetics Univ. Texas Publ. 7213, pp. 145–153.Rogers, 1972), clustered using the Unweighted Pair Group Method of Analysis (UPGMA). The cophenetic correlation of the dendrogram was 0.989.
To resolve underlying genetic affinities and gain a better understanding of population structure, recent effort has focused upon analysis of genetic markers. Surveys of populations of Caulerpa from the central Great Barrier Reef (GBR) in north-east Australia have demonstrated that allozymes provide a useful means for determining species boundaries in the genus (Benzie et al., 1997). The allozyme patterns tended to support previous taxonomic distinctions based on morphology, with genetic differentiation being an order of magnitude greater between species than among different populations of the same species.
Different opinions have been expressed for many years on the taxonomic disposition of Caulerpa taxifolia (Vahl.) C. Agardh. Specifically, the debate is whether C. taxifolia is an ecomorphic variant (an ecad) of another species, C. mexicana Sonder ex Kützing (Jaasund; Coppejans and Coppejans), or a well-defined species in its own right ( Calvert and Silva). According to most accepted taxonomic criteria, ecomorphic variants of C. taxifolia can, under certain conditions, possess the morphological characteristics attributed to C. mexicana, and vice-versa (Chisholm and Olson). Although there appear to be stable differences between west Atlantic representatives of C. mexicana and Mediterranean representatives of C. taxifolia (Chisholm, J.R.M., Jaubert, J.M. and Giaccone, G., 1995. Caulerpa taxifolia in the northwest Mediterranean: introduced species or migrant from the Red Sea?. C. R. Acad. Sci. Paris, Sciences de la vie 318, pp. 1219–1226. View Record in Scopus | Cited By in Scopus (20)Chisholm et al., 1995), the lack of unambiguous morphological criteria suggests that a genetic approach is needed to delineate the two.
C. taxifolia exhibits variation in morphology in different parts of its geographical range. In the central GBR, representatives of C. taxifolia tend to be fine in form, with narrow stolons, fronds and pinnules. In contrast, representatives from the Mediterranean tend to be robust, with heavy stolons and long, broad fronds bearing large pinnules. Given the large geographical separation of Australian and Mediterranean populations, and the differences in their respective tropical and temperate environments, their morphological differences may not be surprising. However, populations of C. taxifolia in southern Queensland, at Stradbroke Island, which experience environmental conditions more in keeping with the Mediterranean, express a range of morphologies that include plants with heavy stolons and long, broad fronds bearing large pinnules.
the extent of genetic variation among widely separated populations of C. taxifolia with similar morphology (the robust form in Australia and the Mediterranean). Given past suggestions that C. taxifolia could be an ecomorph of C. mexicana, two populations of C. mexicana from the Caribbean and two samples (all that could be found despite extensive searches) from the Mediterranean were also included in the analysis to assess their relationships. This analysis represents the first genetic survey of any Caulerpa species over such a broad geographical range.
Anatomy
Caulerpa is bright green with leafy (fern-like) fronds that extend upward from each main stem. Each stem can grow up to nine feet in length.
- Caulerpa taxifolia is a siphonalean alga
- the Mediterranean strain of Caulerpa taxifolia is somewhat different, chiefly in size, length, growth rate and temperature tolerance from samples collected in tropical areas (Boudouresque et al, 1995)
- fronds are feather-like "leaf blades" each of which has a relatively wide central axis (rachis), from which grow many pinnules
- primary fronds grow directly on the stolons at regularly spaced intervals; fronds may be quite short or even absent in shallower water (leaving only the stolons), becoming longer in deeper water in low light conditions; primary fronds are 2-15 cm (1-6 in) in the tropical version of the alga, while primary fronds of the Mediterranean strain range from 5 cm in shallower water, to 40 cm at depths of 15 m, and even to 60-80 cm long (24 in to 38 in) at greater depths (Meinesz, 1995); branching fronds grow from the primary fronds
- pinnules are up to 1 cm long; number 4 to 7 per cm along each side of the frond axis; are usually upcurved, tapering at the ends; some pinnules are split in two at the ends (bifurcate); pinnule spacing and length depend on light availability (Meinesz, 1995)
- stolons (stems) bear the fronds and the rhizoids; stolon length averages 1 to 1.5 m in autumn (Meinesz, 1995); new stolons arise from old stolons that have survived the winter; cumulative stolon length "tends to stabilise around an equilibrium value of 220 m m2" (Meinesz, 1995)
- unlike vascular plants, there are no "roots" on algae; however in C. taxifolia, regularly spaced "rhizoid pillars" descend from the stolons, tapering at the ends, having many extremely thin filamentous "rhizoids"; the rhizoids mimic roots by attaching to rocks and other substrata and taking up and translocating inorganic and organic nutrients from the substrate; "on rock, the lacework of these rhizoids, trapping grains of sand or mud, may form a felt, completely covering the substrate" (Meinesz, 1995; Chisholm et al., 1996)
Life History
In the late 1970s this species attracted attention as a fast-growing and decorative aquarium species that became popular in the saltwater aquarium trade. A clone of the species was cultured for display at the Stuttgart Aquarium in Germany and provided to aquariums in France and Monaco. Around 1984 this species apparently escaped or was released from an aquarium into Mediterranean waters, and rapidly spread from an initial patch of about one square yard to over two acres by 1989. Meinesz reports that by 1997 it blanketed more than 11,000 acres of the northern Mediterranean coastline and has recently been reported off northern Africa. Genetic analysis suggests that all Caulerpa taxifolia plants in the Mediterranean are clones of the original, inadvertently released saltwater aquarium plant.
The Mediterranean strain of Caulerpa was prohibited under the U.S. Federal Noxious Weed Act in 1999. Since then, importation into the United States, as well as interstate transport and sale, including via the Internet, has been a federal offense. In 2001, the California legislature banned the possession, sale, importation, transportation and release of C. taxifolia and eight similar-looking Caluerpa species in California. The city of San Diego recently banned the possession and sale of the entire Caulerpa genus.
Global Invasive Species Specialist Group categorized C.taxifolia among the 100 most "Worst Invasive Alien Species" threatening biodiversity.(1.0aISSG, 2004)
This species has recently been reported near Sydney, Australia, smothering seagrass beds in a manner reminiscent of the invasion in the Mediterranean. Despite bans on its possession in France, Spain, and Australia, this organism continues to be transported and sold by the aquarium trade; fearing its eventual introduction into US waters, over 100 prominent scientists petitioned the federal government in 1998 to ban the use of Caulerpa taxifolia in American aquaria, leading to its designation in 1999 as a prohibited species under the Federal Noxious Weed Act. The discovery of this species in southern California, recently reported in the journal Nature to be genetically identical to the strain in the Mediterranean, confirms that it nevertheless continues to invade marine ecosystems, such as the ecologically rich eelgrass beds that thrive in many of our coastal lagoons. It is likely that the alga was released from an aquarium at the locations in California where it has been discovered, a practice banned under California law.
Locomotion
Mid range spread of this species is easily achieved by currents, which transport fragments of it into new areas yet to be colonized (1.3Chisholm et a., 1997)
Apart from shipping vectors, long range dispersal of this alga was facilitated by the aquarium trade (1.7Schaffelke et al., 2002).
It is capable of growing up to one inch per day, and can survive up to ten days out of water.http://www.ridnis.ucdavis.edu/Caulerpataxifolia.html
Senses
Intelligence
Caulerpa taxifolia is that it grows so fast that it extracts toxic metals and other less usable elements from its environment.
Behavior
Plant defense strategy is usually a result of trade-offs between growth and differentiation (i.e. Optimal Defense Theory – ODT, Growth Differentiation Balance hypothesis – GDB, Plant Apparency Theory – PAT)Plant defense strategy is usually a result of trade-offs between growth and differentiation (i.e. Optimal Defense Theory – ODT, Growth Differentiation Balance hypothesis – GDB, Plant Apparency Theory – PAT)
in the Mediterranean, Caulerpa taxifolia invades the dominant seagrass, Posidonia oceanica, and in invaded areas able to kill up to 45% of Posidonia shoots in one year (Villele and Verlaque, 1994)
Reproductive
Caulerpa spreads readily via fragmentation, making prevention of spread and mechanical removal nearly impossible. Any small fragment of this seaweed has the potential to start a new colony.
Defense
It is capable of growing up to one inch per day, and can survive up to ten days out of water http://www.ridnis.ucdavis.edu/Caulerpataxifolia.html
Chemical
Caulerpa species are known to contain psychoactive substances such as caulerpin, caulerpicin and caulerpenyne. Caulerpa taxifolia species, which can be strongly hallucinogenic.
Caulerpa taxifolia is toxic to herbivores such as sea urchins and fish; where the plant is the sole food source, then these herbivores are eliminated
- Caulerpa taxifolia protects itself by producing substances that are toxic to the Mediterranean's two main macro-herbivores, sea urchins and their eggs (as well as to hamsters and mice) (Lemee et al., 1993), and the fish Sarpa salpa
- toxicity is highly seasonal: highest in July-November, lowest in March-April
Ichthyoallyeinotoxism, or Hallucinogenic fish poisoning, comes from eating certain species of fish found in several parts of the tropics, notably the Indian Pacific. The effects of eating ichthyoallyeinotoxic fishes are reputed to be similar in some aspects to LSD. Experiences may include vivid auditory and visual hallucinations. This has given rise to the collective common name "dream fish" for ichthyoallyeinotoxic fish.
list of psychotropic fish:
- Abudefduf septemfasciatus (Sergeant major) Pacific Ocean, Africa
- Epinephelus corallicola (Grouper) Pacific Ocean
- Kyphosus cinerascens (Bluefish) Indonesia
- Kyphosus vaigiensis (Brass bream) Indonesia
- Mugil cephalus (Flathead mullet) The tropics
- Mulloidichtys samoensis (Golden goatfish) Indonesia
- Neomyxus chaptali (Mullet) Indonesia
- Saganus oramin (Rabbitfish) Indonesia, West Africa
- Upeneus arge (Goatfish) Indonesia
Of the species most commonly claimed to be capable of producing this kind of toxicity include several species from the Kyphosus genus, including Kyphosus fuscus, K. cinerascens and K. vaigiensis.
Toxicology
Caulerpenyne (CYN) is known to induces neurological disorders (i.e. amnesia, vertigo, and hallucinations, reported by 4.59De Haro et al., 1993) on patients with food poisoning due to the ingestion of Sarpa salpa that fed on C.taxifolia. 4.59 DeHaro L., Treffot M.J., Jouglard J., Perringue C., 1993; Trois cas d'intoxication de type ciguateresque aprés ingestion de sparidae de Mediterranée; Ictyol. Physiol. Acta 16:133-146
Caulerpin an indole alkaloid, a red pigment, a hydrophobic macromolecule containing a cyclo-octatetraene ring pigment CAS Registry No. 26612-48-6
Caulerpin and caulerpicin are sometimes referred to as toxins, although they appear to be primarily growth regulators present in various species of macroalgae. They are not particularly toxic to animals or bacteria in most studies.
http://www.reefkeeping.com/issues/2004-10/rhf/index.php
Social Interactions
Ecology
Habitat & Range
Species of the marine green algal genus Caulerpa are distributed between the latitudes of 48°S (Hay et al., 1985) and 45°N (Meinesz, A. and Boudoureseque, C.-F., 1996. Sur l’origine de Caulerpa taxifolia en Méditerranée. C. R. Acad. Sci. Paris, Sciences de la Vie 319, pp. 603–613. View Record in Scopus | Cited By in Scopus (34)Meinesz and Boudoureseque, 1996). Although individual plants are composed of only one cell, most species have complex morphologies, composed of pseudo-organs that often outwardly resemble the roots, shoots and leaves of higher plants. The Mediterranean plants grow on many types of substrates including rocks, sand, mud and dead rhizomes of seagrasses. "Its high growth rate, its total substrate occupation, its improved light access, the increased sedimentation rates it creates, and the synthesis of toxic secondary metabolites (mono- and sesqui-terpenes)" are reasons why Caulerpa taxifolia proliferates and replaces native seaweeds and seagrasses. New colonies usually appear between 2 and 10 meters depth (Meinesz et al., 1993). Growth is highest in summer and fall and C. taxifolia is able to withstand severe extreme limitation (Delgado et al., 1996), adding to its resilience. In specific to temperature tolerance, its lethal minimum temperature in the Mediterranean is 7o C (45o F), lethal minimum temperature elsewhere is 14o C (57o F) (Komatsu et al., 1994); optimum growth temperature is 20-30o C (68-86o F):; its lethal maximum temperature is 32o C (90o F). It has a very low light compensation point allowing existence in extremely low light conditions (Gacia et al., 1994; Komatsu et al., 1994) DNA testing confirmed the 2000 discovery of the Mediterranean strain of Caulerpa taxifolia in Carlsbad and Huntington Harbour, California. This alga can easily spread from these infested areas to other U.S. waters, and because of its robustness, there has been a call for "rapid eradication" (Jousson et al., 2000).
Invasion Threat
It is capable of growing up to one inch per day, and can survive up to ten days out of water. Any small fragment of this seaweed has the potential to start a new colony. This seaweed has been observed to survive many months in 50° F water. Given this tolerance to cold and the remarkable adaptability that this species has displayed, it would be wise for even more northern regions to be aware of the damage that introduction of this species could cause to their native ecosystems.
Global Invasive Species Specialist Group categorized C.taxifolia among the 100 most "Worst Invasive Alien Species" threatening biodiversity.(1.0aISSG, 2004)
This alga poses a substantial threat to marine ecosystems Southern California, particularly to the extensive eelgrass meadows and other benthic environments that make coastal waters such a rich and productive environment for fish and birds. The eelgrass beds and other coastal resources that could be directly impacted by an invasion of Caulerpa are part of a food web that is critical to the survival of numerous native marine species including the commercially and recreationally important spiny lobster, California halibut, and sand basses. However, this threat is not exclusive to California. Aside from the likelihood that this invasive strain could thrive in other warm locales, such as the Gulf of California, the Gulf of Mexico, and the Pacific coast of Florida, cooler waters should not be ruled out as at risk also. This seaweed has been observed to survive many months in 50° F water. Given this tolerance to cold and the remarkable adaptability that this species has displayed, it would be wise for even more northern regions to be aware of the damage that introduction of this species could cause to their native ecosystems.http://swr.nmfs.noaa.gov/hcd/CAULERPA.htm
Prevention
The most important task is to prevent the introduction of ANY aquarium organisms into water bodies. Extreme care must be taken when cleaning or dismantling fish tanks, because a half-inch piece of Caulerpa taxifolia that is inadvertently washed into the gutter while rinsing a fish tank on the lawn could quite plausibly travel through the storm drain directly to a nearby estuary or beach and establish itself there. Aside from caution, an even more responsible action would be to eliminate any risk of accidental introduction by discontinuing the use of Caulerpa in home aquaria. Caulerpa can be removed from the tank, with all the material it is attached to (rocks, gravel, etc), placed in a freezer for 24 hours, and then placed in the trash for disposal in a landfill. Under no circumstances should any unwanted aquarium plants or animals be released into the wild.
It is crucial that all people who spend time exploring the ocean bottom be educated and involved in detection and reporting. SCUBA and free divers as well as recreational and commercial fishermen can participate in the surveillance effort by familiarizing themselves with the appearance and habit of this seaweed.
Can we stop "killer algae" from invading Florida?, by C. Jacoby & L. Walters is a 2 page illustrated fax sheet describing Caulerpa, what it is, what it looks like, why it is invasive, and how people can help prevent an invasion of this noxious aquatic weed.
Response & Intervention
Although delays in recognizing the true threat of the invasion in the Mediterranean make the eradication of Caulerpa taxifolia there unlikely, distribution of the Caulerpa discovered in California is restricted enough that eradication efforts have been optimistically undertaken. After exploring techniques such as dredging, hand removal, draining of the lagoon, and application of various herbicides, a biological consulting firm in San Diego developed and implemented a plan to treat the seaweed in situ to avoid further fragmentation and spread. Each patch of Caulerpa was covered with a heavy plastic tarp that was sealed to the bottom at the edges and fitted with a small "port" on top that allowed for the introduction of herbicide under the tarp. The tarp allowed for the direct treatment of the target patch, while preventing the loss of herbicide to the lagoon waters. Although the algae appeared to have been effectively treated, the tarps were left in place to prevent the growth of Caulerpa from portions of it that grow in the mud and that may not have been fully treated by the herbicide application. All known Caulerpa has been treated in Carlsbad, and the site is surveyed monthly, with monitoring continuing for at least five years in order to detect regrowth. A very similar eradication is currently ongoing in Huntington Harbour.
If Caulerpa taxifolia is observed in the wild, DO NOT DISTURB IT. Note as much information as possible about the location where it was found and report it immediately to the Southern California Caulerpa Action Team at (858) 467-2952, visit the website http://caulerpa.cjb.net or E-mail: caulerpa@rb9.swrcb.ca.gov
Trophic Level & Foraging Behavior
Prey
Caulerpa taxifolia
Predators
Acanthuridae family, specifically the Tanga type of saltwater fish, can subside on almost only this algae and scarfs down caulerpa taxifolia like it is going out of style ! They do well with it in their diet, though they have an adaptation that allows them to metabolize the toxins in the algae.
In the Philippines, where the taxifolia strain grows naturally, people eat the seaweed and enjoy the slight stinging sensation caused by its chemical defense system.http://www-csgc.ucsd.edu/STORIES/Caulerpa.html
Unique Behavior
Caulerpa taxifolia
Scientific Studies
It easily proliferates vegetatively via fragmentation aided by subsequent dispersal via anchors and fishing nets (1.4Meinesz, 1992), or dumping ballast water across the oceans; in particular at harbors, marinas and other places where boats anchor (1.6Boudouresque et al., 1995).
The numbers of individuals of Mollusca, Amphipoda and Polychaeta in Caulerpa taxifolia meadows is greatly reduced (Bellan-Santini et al, 1996)
Prolific growth of Caulerpa along the Cote d’Azur (France), where the introduction was first reported, has been associated with urban wastewater pollution (1.3Chisholm et al., 1997)
Feeding Electivity Indices in Surgeonfish (Acanthuridae) of The Florida Keys. G. Christopher Tilghman, RuthEllen Klinger-Bowen and Ruth Francis-Floyd, Aquarium Sciences and Conservation. Volume 3, Numbers 1-3, 215-223, DOI: 10.1023/A:1011338716923
Caulerpin, caulerpicin, Caulerpa scalpelliformis: comparative acute toxicity study. Vidal, J. P.; Laurent, D.; Kabore, S. A.; Rechencq, E.; Boucard, M.; Girard, J. P.; Escale, R.; Rossi, J. C. Fac. Pharm., Univ. Montpellier I, Montpellier, Fr. Botanica Marina (1984), 27(12), 533-7.
Caulerpenyne, a toxin from the seaweed Caulerpa taxifolia, depresses afterhyperpolarization in invertebrate neurons. Mozzachiodi R, Scuri R, Roberto M, Brunelli M. Department of Physiology and Biochemistry 'G. Moruzzi', University of Pisa, Via S. Zeno 31, 56127, Pisa, Italy.