House Mouse
From Comparative Physiology of Vision
| House Mouse | |
|---|---|
 |
|
| Conservation status | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Rodentia |
| Family: | Muridae |
| Subfamily: | Murinae |
| Genus: | Mus |
| Subgenus: | Mus |
| Species: | M. musculus |
| Binomial name | |
| Mus musculus Linnaeus, 1758 |
|
| Subspecies | |
Contents |
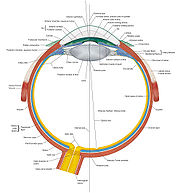
General Anatomy - I'm Stealing Your Cheese and Co-expressing
| Species | Eye Diameter | Rod / Cones | Cone Types | Pupil Shape | Multifocal Optics |
|---|---|---|---|---|---|
| Mus musculus | 5mm | ~97% / ~3% | Two - M and S (UV) | Circular | Yes |
Overall, the visual sensitivity of mice was found to be remarkably similar to that of the human peripheral retina.(7)
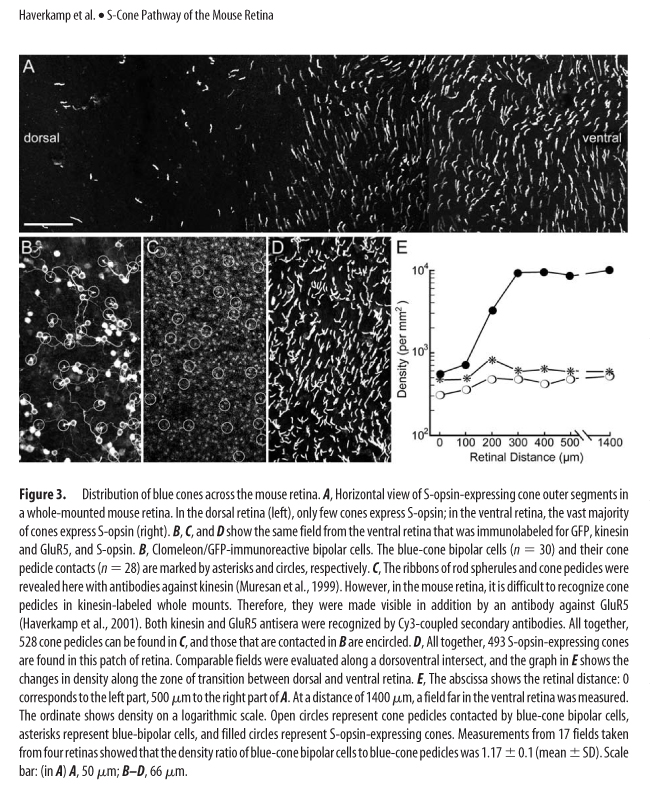
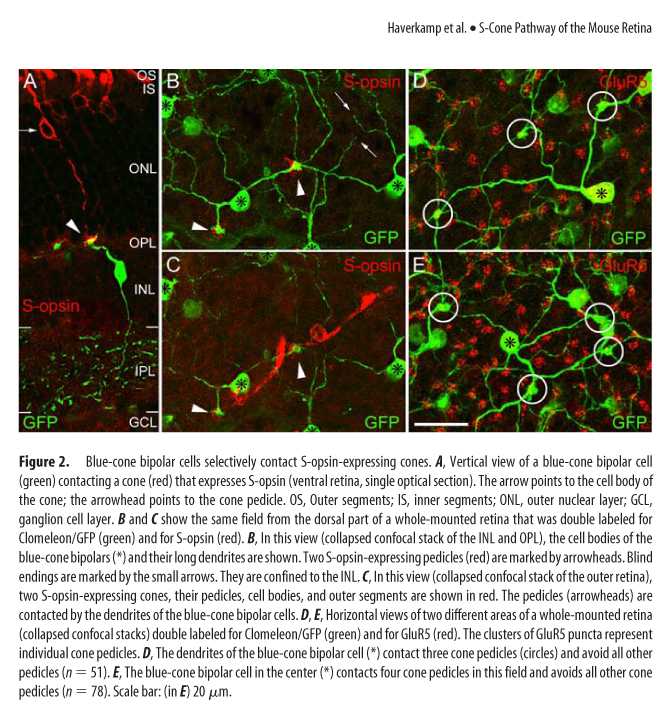
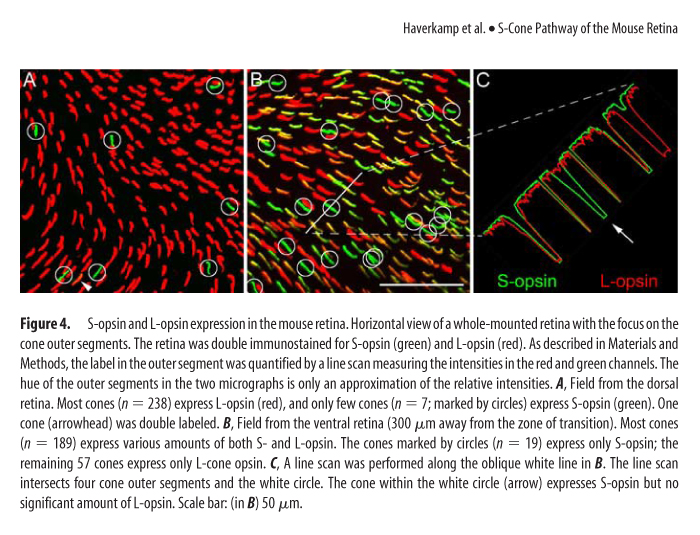
One of the world's most abundant rodents, a common household nuisance and a model species, has a most atypical cone photoreceptor organization. A vast majority of cones present in the mouse retina co-express both UV and M opsins. Only in the middle and ventral retina can you find minimal cones with selective expression of UV opsin and in the far dorsal region cones expressing M opsin. (See Figure 3 and 4, Haverkamp, see below in UV Vision)
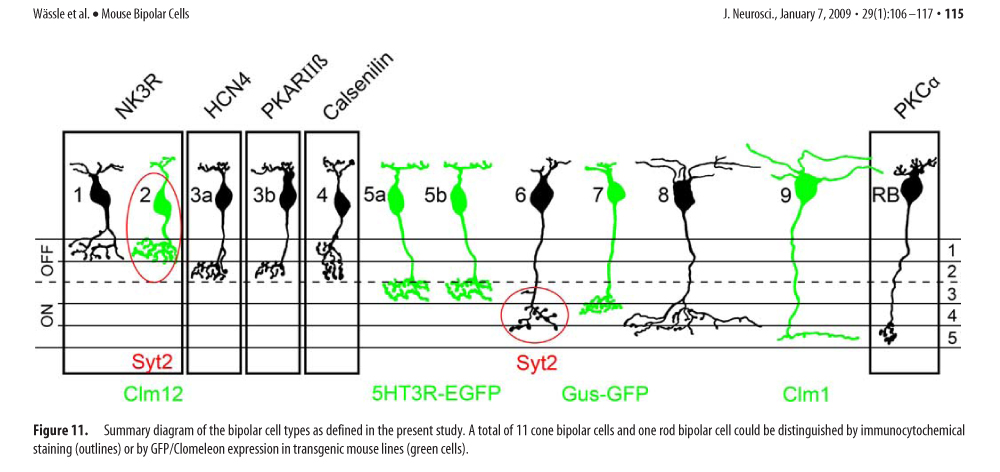
Basic Circuits of Mouse Vision
“daylight” [cone -> cone bipolar]
“twilight” [rod -> cone -> cone bipolar]
“starlight” [rod -> rod bipolar -> AII amacrine -> cone bipolar]
auxiliary rod pathway [rod -> B2 OFF bipolar ->]
One key difference between rods and cones is that the rod disks that contain the photo pigment are completely internalized and separate from the plasma membrane, whereas the cone disks are exposed and immersed in the plasma membrane. This exposure provides a much larger surface area for rapid dynamic adaption. In both cases rods and cones continually renew their outer segments from the base of the outer segment toward the apical end. In order to maintain a consistent outer segment length the rate of renewal and disposal are kept roughly equal.
"The universal and perhaps most fundamental characteristic of cone-based vision in vertebrates is that cones allow the organism to see under daytime illumination, when rod responses saturate."(7)
Under simulated daylight conditions, well beyond rod saturation, WT mice were able to easily perform a basic detection task, demonstrating that they clearly saw well using only cone signals. Interestingly, the study also demonstrated that the cone vision system of the mouse actually takes over at a lower luminance than that of a human, lending the mouse sensitivity during the crucial periods of low light at dawn and dusk. (7)
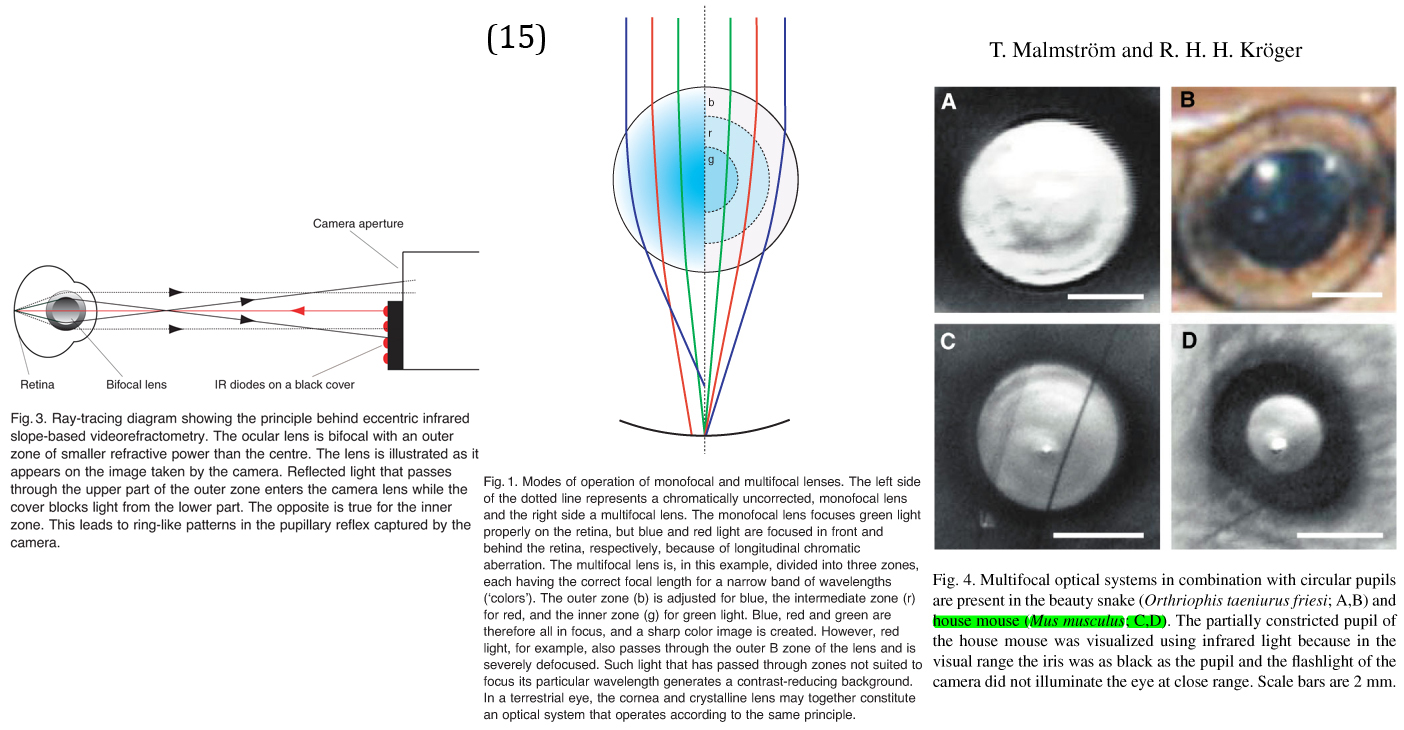
Unique Visual Optics - The Multifocal House Mouse
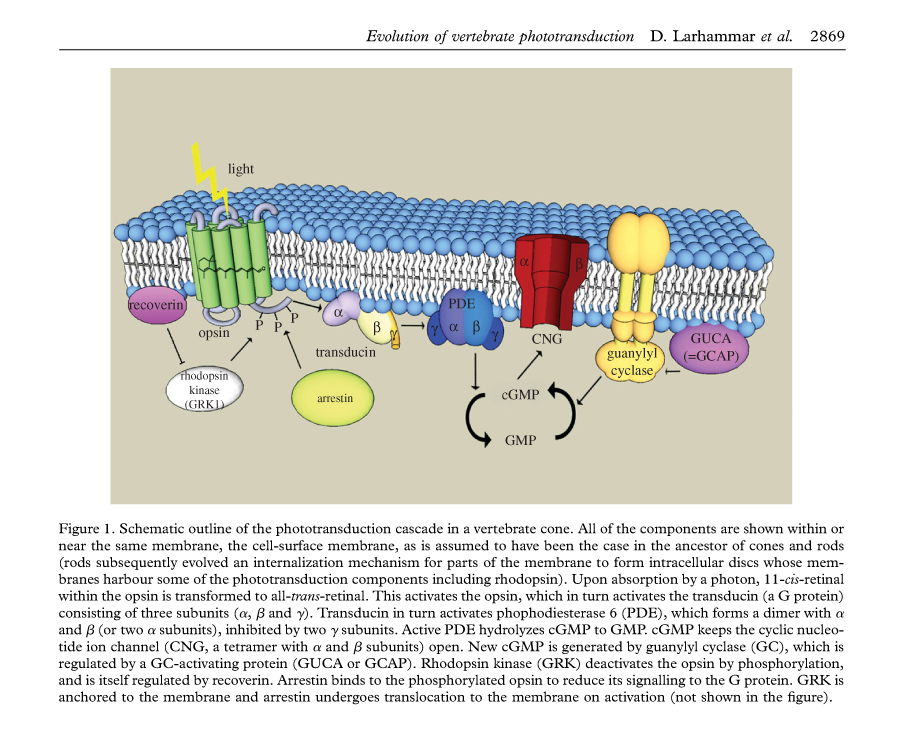
Photo Transduction
Color Vision
The common house mouse does have some capacity for dichromatic color vision, despite evidence for widespread coexpression of both UV and M pigments in cones. The extent to which this coexpression occurs has been shown to vary across the retina in order for the mouse to exploit the differences in spectral absorption of the cones. The house mouse, in comparing the signals from a small number of cones with different absorption spectra, is able to sufficiently discriminate different colors. (9)
UV Vision
Motion Detection
Peering out from the confines of their small, dark subterranean or perhaps suburban habitat, small rodents like the house mouse rely on faint amounts of light to avoid predators. Particularly at dawn and dusk, the motion of faintly backlit silhouettes dominate their visual field, most effectively detected by a rod-driven OFF system. (Yoshihiko Tsukamoto, 4)
Neuronal Processing
Evolutionary Significance
“The separation of the L and S-cone pigments occurred - 500 million years ago and thus represents the phylogenetically ancient, primordial color system (Mollon, 1989).” (H. W. Silke Haverkamp)
UV vision in vertebrates is determined by eight specific amino acids in the S-cone pigment. Contemporary UV pigments in vertebrates retained their UV sensitivity by retaining most of these eight critical amino acids in the UV pigments. The evolution of violet pigments from ancestral vertebrate UV pigment requires a change in at least two of these critical amino acids. Throughout vertebrate evolution a wide range of species have maintained UV vision, and many others have exchanged UV for violet. The use of UV pigments by Mus musculus can be strongly associated with its light environments and behaviors.
Vertebrates, who retained UV vision, use this for such basic behaviors as foraging, mate choice and communication. The presence of UV pigment in a number of rodents and the high UV reflectivity of fresh urine suggests the potential for a selective advantage for mate selection and communication under special circumstances. In the case of foraging, compared to organisms exhibiting violet vision, those with UV can detect UV-reflecting objects much quicker. The selective advantages induced by UV vision can also lead to disadvantages, loosing precision and possessing a greater sensitivity to retinal damages caused by UV light. (3)
References
1 Yau, Yingbin Fu and King-Wai. "Phototransduction in mouse rods and cones." Pflugers Arch. (2007): 805-819.
2 Yokoyama, Yongsheng Shi and Shozo. "Molecular analysis of the evolutionary significance of ultraviolet vision in vertebrates ." PNAS (2003): 8308-8313.
3 Yongsheng Shi, F. Bernhard Radlwimmer, and Shozo Yokoyama. "Molecular genetics and the evolution of ultraviolet vision in vertebrates." PNAS (2001): 11731–11736.
4 Yoshihiko Tsukamoto, Katsuko Morigiwa, Mika Ueda, and Peter Sterling. "Microcircuits for Night Vision in Mouse Retina." The Journal of Neuroscience (2001): 8616–8623.
5 Chang-Jin Jeon, Enrica Strettoi, Richard Masland. "The Major Cell Populations of the Mouse Retina." The Journal of Neuroscience (1998): 8936-8946.
6 Dan Larhammar, Karin Nordstrom and Tomas A. Larsson. "Evolution of vertebrate rod and cone phototransduction genes ." Philosophical Transactions of The Royal Society B (2009): 2867–2880.
7 Frank Naarendorp, Tricia M. Esdaille, Serenity M. Banden, John Andrews-Labenski, Owen P. Gross, and Edward N. Pugh Jr. "Dark Light, Rod Saturation, and the Absolute and Incremental Sensitivity of Mouse Cone Vision." The Journal of Neuroscience, (2010): 12495–12507.
8 Gary A. Williams, Gerald H. Jacobs. "Cone-based vision in the aging mouse." Vision Research (2007): 2037–2046.
9 Gerald H. Jacobs, Gary A. Williams, John A. Fenwick. "Influence of cone pigment coexpression on spectral sensitivity and color vision in the mouse ." Vision Research (2003): 1615–1622.
10 Heinz Wassle, Christian Puller, Frank Muller, Silke Haverkamp1. "Cone Contacts, Mosaics, and Territories of Bipolar Cells in the Mouse Retina ." The Journal of Neuroscience (2009): 106 –117.
11 Krishna K. Ghosh, Sascha Bujan, Silke Haverkamp, Andreas Feigenspan, and Heinz Wassle. "Types of Bipolar Cells in the Mouse Retina." THE JOURNAL OF COMPARATIVE NEUROLOGY (2004): 70-82.
12 Kröger, Tim Malmström and Ronald H. H. "Pupil shapes and lens optics in the eyes of terrestrial vertebrates." The Journal of Experimental Biology (2006): 18-25.
13 Neitz, Maureen Neitz and Jay. "The uncommon retina of the common house mouse." TRENDS in Neuroscience (2001): 248-249.
14 Silke Haverkamp, Stylianos Michalakis, Ellen Claes, Mathias W. Seeliger, Peter Humphries, Martin Biel, and Andreas Feigenspan. "Synaptic Plasticity in CNGA3-/- Mice: Cone Bipolar Cells React on the Missing Cone Input and Form Ectopic Synapses with Rods ." The Journal of Neuroscience (2006): 5248-5255.
15 Olle E. Lind, Almut Kelber and Ronald H. H. Kröger. "Multifocal optical systems and pupil dynamics in birds." The Journal of Experimental Biology (2008): 2752-2758.